Abstract
Urbanization has been a global phenomenon producing great environmental and ecological challenges including species composition shifts in urban areas. To evaluate the interaction networks of the inhabitant metacommunities in urban parks, we applied the nestedness analysis using ecological survey data of birds, reptiles, frogs, and butterflies among 16 parks in Taipei City of Taiwan and identified critical environmental factors for biodiversity conservation. Results found significant nestedness of the metacommunities among these parks, which indicated strong interaction networks and the importance of urban parks for sustaining these metacommunities. In addition, various types of land use had different effects on the species incidence of birds, reptiles, frogs, and butterflies. It demonstrates that landscape design should consider the taxonomic differences in the development of conservation guidelines. Results also showed that the area of parks and the area of woodlot in the park were critical elements for the nested structure. Nonetheless, abundant bird generalists were found to be highly related to the area of buildings within parks, distance to forests, rivers, and the neighbouring parks. Moreover, exotic species were found to affect the interaction networks of native species. However, based on the current information, we cannot confirm the actual effects of the exotic species on the native assemblages. Reptiles and frogs appeared to be more easily threatened by land development and habitat fragmentation due to their lower vagility. In this regard, we suggest that conservation action should focus on preserving green space with large areas and constructing green corridors, creating crucial elements, and increasing the vertical complexity in the parks for species with various vagilities. We believe these strategies can help reduce the impact of urbanization to retain healthy interactions and connections of metacommunities among habitats for effective biodiversity conservation in urban areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization has been known to result in a series of economic, social, and environmental changes leading to various ecological impacts, and is still undergoing (Fan et al. 2019; United Nations 2019). In particular, serious environmental deterioration occurs resulting in fragmentation and reducing habitat connectivity (Danielle et al. 2011) that further change the inhabitant species composition and distribution (Habel et al. 2019), and affect species abundance, compositional variation, ecological integrity, and biodiversity (Alexandre et al. 2008; Danielle et al. 2011). Although some species may have adapted to the niches provided by these urban environments, or generalized species often quickly occupy new niches even in the highly urbanized areas (Čeplová et al. 2017; Apfelbeck et al. 2020), species with habitat specificity or sensitivity to environmental changes suffer serious survival threats (Dennis and Eales 1997). Nowadays, there is a general awareness of the importance of green space for species survival and biodiversity conservation (Lepczyk et al. 2017), so the idea of “wildlife-inclusive urban design” has been recently promoted and incorporated into urban planning that gives much value to the green space like parks and yards within the urban areas (Apfelbeck et al. 2020).
To explore the importance of green space to the state of ecological communities, the concept of nestedness proposed by Darlington (1957) has been used to describe the distribution pattern of animals (Pinheiro et al. 2019; Tan et al. 2021). The nestedness depicts the mechanism that drives the biogeographical pattern. In addition, green space in urban areas is often situated apart, like an ecological island. Based on the concept of island biogeography, the richness of species is distributed in association with the migration and extinction probability determined by the size and the isolation level of the correspondent habitats (MacArthur and Wilson 2001). The species presented in species-poorer islands are often a subset of the species composition in species-richer islands (Atmar and Patterson 1993). Since the early development of the concept of nestedness (Darlington 1957), numerous studies have started to quantify the nested patterns and structures (Atmar and Patterson 1993; Cutler, 1994; Azeria and Kolasa 2008; Wang et al. 2010; Pinheiro et al. 2019). The “Weighted nestedness based on the overlap and decreasing abundance” (WNODA) has been applied as an advanced quantification method to detect the nested structure and standardize the bias resulting from the size difference of the species composition matrix. Specifically, the WNODA calculates the predictive gradient of the species composition matrix as the nestedness. It considers the inequality and overlap of the internal species composition and conservatively presents the abundance weighted matrix, it resolves the rare species influence to examine the habitat-species relationships mimicking the real interactions among the ecological systems of metacommunities and their habitats (Pinheiro et al. 2019). Although WNODA provides a way to evaluate the habitat-species interactions, suitable for detecting the metacommunity interactions in isolated habitats (Atmar and Patterson 1993), studies showed that selective extinction/colonization, habitat nestedness, and passive sampling also determine the level of nestedness (Atmar and Patterson 1993; Vázquez et al. 2007; Wang et al. 2010). Besides, environmental conditions and landscape design are important considerations beyond the nestedness for species conservation.
This study aims to explore the importance of urban green space for biodiversity conservation and to investigate how different environmental factors affect the species composition and connectivity between metacommunities in urban areas. In this paper, we apply the WNODA to examine the nestedness of metacommunities interaction networks among the selected 16 parks in the highly urbanized Taipei city of Taiwan. We first test the random placement hypothesis and then apply the nestedness analysis to evaluate the temporal changes in the nestedness. Following that, we examine the relationship between gradients of environmental factors and species incidences from nestedness by Spearman’s correlation test (Schouten et al. 2007; Ulrich 2009). In the end, we list trade-offs between land development and biodiversity conservation and provide considerations and suggestions for park design and urban planning.
Methods
Study area and data description
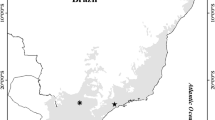
Taipei City (24°N, 121.5°E), the capital of Taiwan, is located in the riverine basin situated in the subtropical monsoon climate zone (Huang 1993). With rapid urbanization, Taipei City has undergone a vast land-use change and changes are continuing (Jim and Chen 2008; Chang et al. 2021). Excluding water fields and wetlands, there are 976 small parks and green spaces with a total area of 1403 ha distributed across Taipei City (Parks and Street Lights Office 2018).
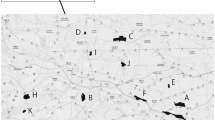
The ecological surveys, provided by the Taipei City Animal Protection Office, have been conducted regularly in parks with an area greater than 1 ha for long-term investigations of birds, reptiles, frogs, butterflies, dragonflies, fish, and aquatic invertebrates from 2007 to 2017. In this study, we assembled species observation data in 16 parks based on the common biogeographic history and the complete species survey over the investigation periods to represent the nearly 1,000 parks in the city (Fig. 1). These 16 parks have been selected under the biodiversity monitoring program by the Taipei City Animal Protection Office, Taipei City Government since 2006 with a standard ecological survey process (Lee et al. 2016). They account for 1.6% of the number of parks in Taipei City but comprise different kinds of environmental settings that occupy 18% of the total area of the parks and green spaces.
To represent the interactions among species and their habitats, we conducted the nestedness analysis with species that occurred in all the 16 parks with the overlapped period from 2008 to 2015. The selected species included metacommunities of bird, reptile, frog, and butterfly. We sorted these species composition data into a species-habitats matrix (Atmar and Patterson 1993) using ArcGIS version 10.5. The species composition data were organized into two datasets. One included the exotic and native species, and the other included only the native species. We arranged the species-habitats matrix with a vertical axis representing the species composition of each habitat, and a horizontal axis the species incidences in all the habitats (Atmar and Patterson 1993; Almeida-Neto and Ulrich 2011) using the software of R 4.0.3. After that, we adjusted the abundance of each species by the frequency of surveys to produce the weighted abundance.
Testing the random placement hypothesis
To examine if passive sampling affects the nestedness analysis, we applied the random placement model to test the nested pattern in species richness and composition by random sampling from a common species pool (Patterson and Atmar 1986; Ulrich et al. 2009). The random placement model assumes that the species are randomly distributed across the sites, so the incidence of a species is proportional to the inhabitant area (González-Oreja et al. 2012). Therefore, the expected number of species per site and the incidence of each species can be derived by the following equations (Coleman et al. 1982):
where \({a}_{p}\) is the area of the pth site, \({\mathrm{\alpha }}_{p}\) is the proportional cover of the pth site to the total area of \({{\Sigma }_{\mathrm{p}}(a}_{p})\), ŝ(α) is the expected number of species per site with summing across bq individuals of qth species, S is the total number of species, and s2(α) is the variance of ŝ(α) to plotting the expected species number of each site with confidence interval. To test if the species community follows the random placement hypothesis, we followed the method developed by González-Oreja et al. (2012) that replaced S with the number of p sites to calculate the expected incidence of each species, and summed across the \({\mathrm{\alpha }}_{p}\) of pth site instead of bq individuals. Then, by plotting the observed richness per site against \({\mathrm{\alpha }}_{p}\), when more than two-thirds of the observation data follow the trends of the expected number of species per site within one standard deviation (i.e., the bands of ŝ(α) ± s(α)), then we can determine the ecological observation data systematically obey the random placement hypothesis. In contrast, if there are excessive observed data departure from the trends, it indicates the observed species community appears a non-random pattern in species richness and composition (González-Oreja et al. 2012).
The nestedness analysis
To perform the nestedness analysis, we constructed a species-habitat matrix by filling in the weighted abundance in a hierarchical relationship, that organized the matrix in order by the magnitude of the marginal sums of the weighted abundance in the rows and columns. As such, parks with higher richness and abundance were placed in the upper rows, and species with higher incidences to the columns at the left (Ulrich et al. 2009). Then, we applied WNODA to examine whether the inhabitant metacommunities of bird, reptile, frog, and butterfly exist in nested patterns and the associated gradient change among the selected 16 parks in Taipei City. The higher the WNODA, the stronger the nested structure (Pinheiro et al. 2019). To permit an unbiased comparison of different size communities, the nested structure of the species-habitat matrix should be sorted and standardized (Pinheiro et al. 2019). To make WNODA independent of matrix size or shape, we measured WNODA by double normalization of the metric of WNODAc and WNODAr (Atmar and Patterson 1993; Almeida-Neto and Ulrich 2011; Pinheiro et al. 2019):
where WNODAc represents the nestedness for columns, WNODAr is the nestedness for rows, m is the number of islands placing in rows, n is the number of species placing in columns, i is the ith column, and j is the next column of the ith. Since column i and column j are paired, \({k}_{ij}\) represents the rows with decreasing values. Therefore, WNODAc is calculated by adding up the declined values of k/n to represent the gradient of species incidence. Similarly, WNODAr is calculated by adding up the declined values of k/m to represent the gradient of species composition (Atmar and Patterson 1993; Almeida-Neto and Ulrich 2011; Pinheiro et al. 2019). The number of “100” was multiplied to standardize WNODAc and WNODAr to the range of the nestedness index from 0 to 100, allowing an unbiased comparison of the metacommunities with different sizes. Based on the characteristics of the original matrix, we produced a null model to perform permutation. We applied the Fixed row totals-Equiprobable column totals as the null model (i.e., the FE null model, Gotelli 2000; Ulrich and Gotelli 2007). It keeps the size and marginal sum to retain the characteristics of the original matrix, but permutates by randomizing species incidence equiprobably to examine the significance level of WNODA (Moore and Swihart 2007; Ulrich and Gotelli 2007; Pinheiro et al. 2019).
Identification of critical environmental factors
As the nestedness is associated with different probabilities of selective immigration and extinction of species (Atmar and Patterson 1993; Vázquez et al. 2007), it can be driven by the interaction of species composition, abundance, and heterogeneous environmental conditions. To identify the critical environmental factors, we allocated potential environmental factors based on the rationale of island biogeography associated with areas and/or distances to the sources of species, including factors of the area or boundary ratio of parks, road or building density around parks, and distance to usable habitats that may affect the survival of species and the interaction networks of the metacommunities (Atmar and Patterson 1993; MacArthur and Wilson 2001; Platt and Lill 2006; Ulrich 2009; Lee et al. 2016). In addition, to evaluate the role of landscape design of the parks to sustain the biodiversity (Rico-Silva et al. 2021), we considered the configuration settings within each park, including landscape components, habitat heterogeneity, and green space within each park. The Landsat-8 satellite image from the USGS Earth Explorer was used to digitalize the chosen environmental factors. Then to select the critical environmental factors, as well as avoid factors with high correlations, we performed a correlation matrix to exclude factors with a correlation coefficient higher than 0.7 and at a significance level of P < 0.05 (Dormann et al. 2013). After that, we examined the influence of the surrounding environmental configuration on the interaction networks of the metacommunities by Spearman’s correlation test considering the relationship between ecological gradients and species incidences from nestedness using the following equation (Ulrich 2009):
where rs is the correlation coefficient, ranging from -1 to 1, v is the order of each object, w is the total number of objects, \({R}_{{x}_{v}}\) and \({R}_{{y}_{v}}\) represent the arrangements of habitats in the matrix with the maximum nestedness, and the selected environmental factors of the habitats of Rx in ordered, to the rank of x and y of the vth object, respectively. After that, we performed a one-thousand-times permutation of Ry and applied two-tailed test to check the statistical significance of rs on each of the environmental factor (Schouten et al. 2007; Wang et al. 2012).
Below we describe the calculation of the potential environmental factors considered in this study.
-
A.
Area of different land-use types within each park.
To estimate the land-use types within each park, including woodlot, shrubs, grasslands, water zone, roads, and buildings, we downloaded a map from Google Maps and performed an in-situ investigation in each park to mark the correct land-use types on the map. Then we digitalized and quantified the projected areas of each land-use type within each park using Arc-GIS version 10.5.
-
B.
Habitat heterogeneity.
We determined the habitat heterogeneity (Harini 2002) by calculating the patch number of each land-use type using the Simpson’s diversity index as (Simpson 1949):
$$\mathrm{D}=1-\frac{\sum_{1}^{i}{u}_{i}{(u}_{i}-1)}{U\left(U-1\right)},$$where D represents Simpson’s diversity index, ui is the number of patches of ith land-use type, and U is the total number of the land-use types.
-
C.
Identification of green space within each park.
To identify the green space within each park, we applied two indices to represent the intensity of live green vegetation, including (1) proportion of green space, and (2) average normalized difference vegetation index (NDVI) within green space. To systematically calculate the two indices, we gathered the satellite images of Taipei City from Landsat-8 from 2013 to 2015 and performed a geometric correction. We only used the satellite images without clouds to reduce the effects of clouds and the derived errors. The NDVI can be calculated as (Rouse et al. 1974):
$$\mathrm{NDVI}= \frac{NIR-RED}{NIR+RED},$$where NIR is the far-red light spectrum value, and RED is the red-light spectrum value.
Based on the calculated NDVI, the “proportion of green space” was determined as to where NDVI > 0 within each park, and “average NDVI within green space” can therefore be examined within the chosen green space.
-
D.
Area and boundary ratio of parks.
We estimated the area of parks using ArcGIS version 10.5. To represent the possible edge effects, we calculated the boundary ratio of parks as the perimeter of the park divided by the area.
-
E.
Road and building density around parks.
Building and road density around parks were determined separately as the proportion of area occupied by buildings or roads within a buffer of 100 m around each park (Rico-Silva et al. 2021).
-
F.
Distance to usable habitats. To examine the ecological connection between usable habitats, like green spaces and aquatic environments, we calculated the shortest distance from each park to the nearest habitats, including forests, rivers, and the neighbouring parks. In this study, forests were defined as an area occupied by trees larger than 5 hectares.
Results
Overview of the composition of the metacommunity
We reviewed the selected biological metacommunities of bird, reptile, frog, and butterfly, and organized the observation data into native versus exotic species. Based on the observation records, there were 10 exotic and 77 native bird species. The exotic bird species included Muscovy Duck (Cairina moschata (Established Feral)), Rock Pigeon (Columba livia), Oriental Magpie (Pica serica), Mallards (Anas platyrhynchos (Domestic type)), Asian Glossy Starling (Aplonis panayensis), Oriental Magpie-Robin (Copsychus saularis), Common Myna (Acridotheres tristis), Javan Myna (Acridotheres javanicus), Black-collared Starling (Gracupica nigricollis), and Parakeet (Conuropsis carolinensis). In the observed 77 native bird species, Eurasian Tree Sparrow (Passer montanus) was found to be the dominant species in the selected 16 parks.
The observed metacommunity of reptiles included 5 exotic and 10 native species, which the exotic species were Red-eared Slider (Trachemys scripta elegans), Cuban Slider (Trachemys decussata), Map Turtle (Graptemys sp.), River Cooter (Pseudemys concinna), and Common House Gecko (Hemidactylus frenatus), whereas Swinhoe's Japalure (Diploderma swinhonis) was the dominant native species in the selected 16 parks.
The observed composition of the metacommunity of frog was found to include 2 exotic and 13 native species. The exotic frog species were American Bullfrog (Lithobates catesbeianus) and Brown Tree Frog (Polypedates megacephalus); while the native species were mainly composed of Asian Common Toads (Duttaphrynus melanostictus) in the selected 16 parks. In terms of the metacommunity of butterflies, all of the 71 recorded species were native species, and Pieris canidia was the dominant native species in the selected 16 parks.
By testing the random placement hypothesis, we found that the observed number of species per site did not follow the expected trend of the random placement model (Fig. 2a). For instance, birds happened to have only one observed species within the band of the expectation, which accounts for only 6.2% of the total observed number of species per site. For butterflies, there were four observed species, about 25%, falling into the band. Reptiles and frogs both had six observed species per site in the band, accounting for about 40% and 37.5%, respectively (Fig. 2a). Nonetheless, the majority of the observed incidents per species followed the expected trend (Fig. 2b). The divergence of the observed incidents per species from the random placement model was 13 (17%) for birds, 3 (4%) for butterflies, 4 (25%) for reptiles, and 3 (27%) for frogs (Fig. 2b).
Comparison of the observed data to the expected trend from the random placement model for testing the random placement hypothesis on the metacommunities of birds, butterflies, reptiles, and frogs. (a) The black solid line represents the expected number of species per site and the grey band discloses the range of ± 1 standard deviation (grey band), and (b) the black solid line represents the expected count per species and the grey band discloses the range of ± 1 standard deviation
The nestedness structure among metacommunities and parks
Excluding the exotic species in the composition data, results showed that all the metacommunities of native species had significant nestedness among these 16 parks in Taipei (Table 1 and Fig. 3). Reptiles appeared the strongest nestedness (WNODA = 60.93, P < 0.001), followed by frogs (WNODA = 51.12, P = 0.017) and birds (WNODA = 48.17, P < 0.001), and butterflies the weakest (WNODA = 28.91, P < 0.001). However, when including the exotic species in the analysis, the nestedness of all the biological groups slightly declined (Table 1 and Fig. 3). Based on the temporal trend of WNODA, we found that the WNODA of frogs and reptiles was usually higher than that of birds and butterflies (Fig. 4). Nonetheless, we tested the trend using the method of ordinary least squares and found none of the taxon’s temporal trends in nestedness reached a significant level of P < 0.05. The correspondent P-value of the temporal trend of nestedness on birds, butterflies, reptiles, and frogs was 0.258, 0.323, 0.393, and 0.471, respectively.
Identification of critical environmental by Spearman’s correlation test
According to the correlation matrix, we excluded some of the highly correlated environmental factors, including the area of grass, roads, and buildings, boundary ratio of parks, and road density around parks (Fig. S1). Analysis results from the Spearman’s correlation test found that the land-use types within parks showed a different level of influence, and the habitat heterogeneity showed lower and non-significant correlations on the nestedness of the metacommunities (Table 2). In particular, we found a significant positive correlation between certain land-use types and the incidence of native species of birds, reptiles, and butterflies. For example, the correlation coefficients of the “area of woodlot in the park” to native birds, reptiles, and butterflies were 0.52, 0.78, and 0.70, respectively. Yet, the correlation coefficient of frogs to the “area of woodlot in the park” was at a lower value (0.27), and not significant. In addition, we also found that shrub is a critical land-use type for birds and butterflies, with a higher positive correlation coefficient of 0.67 for native birds at a significance level of P < 0.01, and for native butterflies with a correlation coefficient of 0.58 at P < 0.05. Furthermore, results found significant correlations of birds and butterflies to the area of parks with a correlation coefficient of 0.54 and 0.47, respectively. Besides, the species incidence of birds was found to be most affected by distance to rivers with a positive correlation coefficient of 0.77 at a significance level of P < 0.01. In contrast, the distance to the neighbouring parks appeared a negative correlation of -0.56 at a significance level of P < 0.05 to the species incidence of birds (Table 2).
More importantly, we found different analysis results of the exotic species on the interaction networks of native species. It seemed that the species incidence gradient of exotic species was more random than that of native species. For example, when including the exotic species in the analysis, the significance level of the correlation of birds to the “area of woodlot in the park” turned from significant to non-significant. An interesting result was found that with the exotic species, the correlation of the building density around parks to birds decreased from -0.41 to -0.34, and the significance level dropped from P < 0.1 to insignificance. Similarly, the area of parks was found to be a significant factor whether we included the exotic reptile species. However, “average NDVI within green space” was the factor that changed the correlation from 0.63 (P < 0.01) to 0.41 (P < 0.1) when the exotic species were included (Table 2).
Discussion and conclusion
To examine the interaction networks of metacommunities of birds, reptiles, frogs, and butterflies among the parks in urban areas, we applied the nestedness analysis of WNODA to measure and observe the community patterns in habitats facing fragmentation or isolation conditions (Atmar and Patterson 1993). Results showed significant nestedness of all the metacommunities among these parks, implying the importance of parks in the urban ecosystems as “island-like” habitats governing the assemblage networks and ecological community interactions. Given the selective colonization and extinction pressure caused by the respective environmental properties of each habitat, the assemblage networks are prone to develop a nested structure with unequal biogeographical patterns of the species distribution and abundance (Atmar and Patterson 1993; MacArthur and Wilson 2001; Vázquez et al. 2007; Wang et al. 2010). As a result, when the system of these ecological islands becomes stable, the ubiquity of nestedness shows (Thebault and Fontaine 2010).
We tested the random placement hypothesis of the metacommunities of birds, reptiles, frogs, and butterflies, and found that the observed species number of birds and butterflies did not obey the expected trend (Fig. 2a), showing the species composition had a nested pattern rather than randomness (González-Oreja et al. 2012). Nonetheless, most of the species sequences followed the expected trends of the random placement model (Fig. 2b), implying the nested pattern might be caused by passive sampling from a common species pool (Patterson and Atmar 1986; Ulrich et al. 2009). However, habitat conditions and sample size of the species could influence the nested pattern. More work is still needed before a definite conclusion can be reached about this point.
Based on WNODA with native species only, reptiles and frogs showed stronger nested structures than birds and butterflies. This may indicate a greater influence of habitat fragmentation on the biological groups with lower vagility being more restricted in their movements (Habel et al. 2019), affecting the species distribution that further shapes the nested pattern (Wang et al. 2010). It also demonstrates the necessity of the conservation strategy of green spaces during land development for those being threatened or struggling to survive, such as the lower vagility biological groups, in the highly fragmented urban environments. However, none of the temporal changes of the four metacommunities’ nestedness showed significant upward trends (Fig. 4), which may be obfuscated by the species distribution affected by seasonality (De la Hera 2019). To strengthen our understanding of the temporal nestedness patterns to the metacommunity composition and the important features of parks, continued species survey and habitat monitoring in different seasons across long-term time scales is required.
When we included both the native and the exotic species in the WNODA calculation, results appeared as declined nestedness of all the four selected metacommunities showing a more disordered interaction network of the associated assemblages among these parks. The declined nestedness implied that the distribution of the exotic species was more random than the native species (Atmar and Patterson 1993). In addition, the niches of the exotic species might be more general than those inhabitant native species to adapt to the urban environments (Čeplová et al. 2017), which might threaten the survival of the native species due to competitive disadvantages (Gaertner et al. 2016). As such, controlling or removing the exotic species is commonly suggested to prevent the territory development for native species conservation (Gaertner et al. 2016). However, it is difficult to partition the effects of exotic species on the native ones, because of the confounding effects caused by interspecific competition, predation, and many other complex interactions (González-Oreja et al. 2018). Therefore, although in our analysis we found that the interaction network of exotic species was more random than that of native species, we cannot confirm the actual impacts of the exotic species on the native assemblages.
Given the importance of parks in sustaining the interaction networks among different metacommunities, we cooperated the Spearman’s correlation test to explore the critical environmental factors and crucial elements in the parks for landscape improvement suggestions (Ulrich et al. 2009). Results found that various types of land use had different effects on the species incidence of birds, reptiles, frogs, and butterflies, demonstrating that the landscape design should consider the taxonomic differences in the development of conservation guidelines. The correlation reflected different importance levels of landscape settings in promoting species incidence (Fleishman et al. 2002), due to the different resource requirements that formed the community structure. For example, the vegetation was found to affect the incidence of butterflies more than that of birds, because the behaviors of butterflies on the pollination, reproduction, and foraging are all directly associated with plants (Fleishman et al. 2002). Consequently, increasing the number of native nectariferous plants in the parks may be an effective way to promote the conservation of butterflies (Oliveira and Scheffers, 2019). Although less correlated than butterflies, vegetation also helps shape a habitat environment for birds on food availability and noise reduction to avoid human disturbances (González-Oreja et al. 2012).
Besides, areas of trees and shrubs within parks were found to be positively correlated with the species incidence of native species of birds, reptiles, and butterflies. This reveals that the design of a multi-layered environment could potentially benefit biodiversity conservation by providing diverse habitats for different species, enhancing the robustness of the ecological interaction networks (Oliveira and Scheffers 2019). The species incidence of native reptiles was found in parks where there were a high variety of vegetation resources offering hiding and inhabiting places (Wang et al. 2010). Therefore, promoting the conservation of native reptiles requires abundant trees and shrubs. Surprisingly, habitat heterogeneity, as a transformed index, did not show significant correlations to birds, reptiles, frogs, and butterflies. We suspect the reason may be from the relatively small scale of the parks in Taipei causing the habitat heterogeneity relatively minor in comparison with other environmental factors (Schouten et al. 2007; Rico-Silva et al. 2021). This highlights the irreplaceable role of the in-situ measurements or observations in understanding reality. It also warns against the use of the transformed index as a supplementary and/or a more comprehensive addition.
In addition to the configuration of parks, the area of and distance to usable habitats, and sources of species are important factors affecting the nestedness (Atmar and Patterson 1993; MacArthur and Wilson 2001). On the landscape scale, “area of parks” exhibited a positive correlation with the species incidence of birds and butterflies significantly. According to the correlation matrix, we observed a negative correlation of “area of parks” to the boundary ratio of parks, and road or building density around parks (Fig. S1). It demonstrates that a larger park with a lower boundary and less isolation may help reduce the edge effects to benefit the incidence of birds and butterflies. Larger areas also help avoid interference to reduce the effects of human disturbances. This is observed in birds’ occurrence or habitat preference that when humans disturb them while foraging, singing, or doing other activities, they tend to hide and/or leave (González-Oreja et al. 2012). The preservation of large green spaces can help provide various niches to accommodate different species and offer a relatively low disturbance space in urban areas for species to survive (Lee et al. 2016; Čeplová et al. 2017). Therefore, it is important to maintain a minimum area of suitable patches during the development (Atmar & Patterson, 1993; González-Oreja et al. 2012).
Moreover, our results found that the species incidence of birds was negatively correlated with distance to the neighbouring parks, and positively correlated to distance to the nearest forests and rivers. It indicates the importance of habitat accessibility for birds to stabilize the interaction networks and increase the re-colonization rates of native species. Loss of connection could potentially impact the networks and resilience of metacommunities and increase the probability of extinction (Alexandre et al. 2008). Actions can be done to uphold the decreasing biodiversity of birds in the highly urbanized environment (Alexandre et al. 2008), including developing green corridors between habitats, improving connections between parks and natural areas (Simberloff and Cox 1987), increasing natural designs, planting native nectars and fruits (Akif et al. 2020), and preserving existing green space. Interestingly, the incidence of birds, either native or exotic, was found to remain a positive correlation with distance to the nearest forests and rivers significantly. This situation did not follow the expectation about the habitat accessibility in the island biogeography (Atmar and Patterson 1993; MacArthur and Wilson 2001) that may be caused by the habits of the generalist species accounting for the majority of total weighted abundance, such as Eurasian Tree Sparrow (Passer montanus) accounted for 33.7% to affect the arrangements of the abundance. Since the ecological requirements for the generalists of birds, often the omnivorous, granivorous, or cavity-nesting birds, are more easily fulfilled in urban areas (Simberloff and Cox 1987), it may represent the indirect impact of urbanization on the richness of native birds with narrow niches (Chace and Walsh 2006). It also implies the revealed community interactions may be an outcome of species selection, and similarly, the abundance-weighted index is influenced by the incidence of common species.
When including the exotic species, we observed that the significance level of some correlations shifted. In terms of land-use types within each park, the factor of the “area of woodlot in the park” turned into non-significant, while the “area of shrubs” holds its positive and significant correlation. Besides, with the inclusion of the exotic bird species, the “density of buildings around the parks” became less negative and not significantly related, reflecting the species-specific adaptability of the exotic birds in the urban areas and their utilization of the roads and buildings. If the thrived population of exotic bird species outcompetes the native bird species, it may cause the loss of biodiversity (Chace and Walsh 2006). Similarly, the species incidence of native reptiles was found to be significantly correlated to “area of woodlot in the park” and “average NDVI within each park”, expressing the need for native reptiles, such as Swinhoe's Japalure and Hekou's Gecko (Gekko hokouensis), for high-quality green vegetation and large trees. However, when the exotic reptile species were included in the analysis, the correlation coefficient between species incidence and “area of parks” became higher and the correlation to “average NDVI within each park” decreased. These changes may be caused by the dominant exotic species of red-eared sliders, an aquatic species that have often been abandoned in large parks and have been found to establish their population there (Zhang et al. 2020). In this regard, these results reinforce the need to control the introduction of exotic species for native species protection.
In conclusion, metacommunities of birds, reptiles, frogs, and butterflies showed significant nestedness among the 16 parks in Taipei, indicating strong interaction networks of the metacommunities among these habitats (Atmar and Patterson 1993). The existence of parks plays a vital role in sustaining these metacommunities (Almeida and Ulrich 2011). In addition, land development may affect the biological groups with lower vagility more seriously, such that in our analysis reptiles and frogs were found to be more easily threatened by habitat fragmentation (Habel et al. 2019). To reduce the impact of urbanization, and help retain healthy interactions and connections of metacommunities among the habitats, we suggest conserving green space with large areas and constructing green corridors (Alexandre et al. 2008). In the parks, the landscape settings affect the species’ incidence differently. The landscape design should consider the taxonomic differences in the development of conservation guidelines. For birds, butterflies, and reptiles, it is recommended to increase the area of shrubs and trees, and plant native nectariferous vegetation. For various species with different vagilities, we suggest increasing the vertical complexity of multi-layered trees to enhance their survival (Oliveira and Scheffers 2019). We believe that these strategies are essential to constructing wildlife-inclusive cities and conserving the community ecology in urban areas.
Data availability
The ecological survey data used in this study may be available with the permission of the Taipei City Animal Protection Office.
Code availability
Not applicable.
Change history
04 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11252-022-01295-8
References
Akif K, Engin E, Sertac K, James TA (2020) Bird diversity along a riparian corridor in a moderate urban landscape. Ecol Indic 118:106751. https://doi.org/10.1016/j.ecolind.2020.106751
Alexandre CM, Rafael GP, Jean PM (2008) Relative effects of fragment size and connectivity on bird community in the Atlantic Rain Forest: Implications for conservation. Biol Conserv 141:2184–2192. https://doi.org/10.1016/j.biocon.2008.06.008
Almeida-Neto M, Ulrich W (2011) A straightforward computational approach for measuring nestedness using quantitative matrices. Environ Modell Softw 26:173–178. https://doi.org/10.1016/j.envsoft.2010.08.003
Apfelbeck B, Snep RPH, Hauck TE, Ferguson J, Holy M, Jakoby C, Jakoby C, Maclvor S, Schär L, Taylor M, Weisser WW (2020) Designing wildlife-inclusive cities that support human-animal co-existence. Landsc Urban Plan 200:103817. https://doi.org/10.1016/j.landurbplan.2020.103817
Atmar W, Patterson BD (1993) The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382
Azeria ET, Kolasa J (2008) Nestedness, niche metrics and temporal dynamics of a metacommunity in a dynamic natural model system. Oikos 117:1006–1019. https://doi.org/10.1111/j.0030-1299.2008.16529.x
Čeplová N, Kalusová V, Lososová Z (2017) Effects of settlement size, urban heat island and habitat type on urban plant biodiversity. Landsc Urban Plan 159:15–22. https://doi.org/10.1016/j.landurbplan.2016.11.004
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007
Chang CR, Chen MC, Su MH (2021) Natural versus human drivers of plant diversity in urban parks and the anthropogenic species-area hypotheses. Landsc Urban Plan 208:104023. https://doi.org/10.1016/j.landurbplan.2020.104023
Coleman BD, Mares MA, Willig MR, Hsieh Y-H (1982) Randomness, area, and species richness. Ecology 63:1121–1133. https://doi.org/10.2307/1937249
Cutler A (1994) Nested biotas and biological conservation: metrics, mechanisms, and meaning of nestedness. Landsc Urban Plan 28:73–82. https://doi.org/10.1016/0169-2046(94)90045-0
Danielle FS, Craig M, Hugh PP, Richard AF (2011) The influence of patch area and connectivity on avian communities in urban revegetation. Biol Conserv 144:722–729. https://doi.org/10.1016/j.biocon.2010.10.014
Darlington PJ (1957) Zoogeography: the geographical distribution of animals. John Wiley & Sons Inc, New York
De La Hera I (2019) Seasonality affects avian species distribution but not diversity and nestedness patterns in the urban parks of Vitoria-Gasteiz (Spain). Anim Biodiv Conserv 42.2:279–291. https://doi.org/10.32800/abc.2019.42.0279
Dennis RLH, Eales HT (1997) Patch occupancy in Coenonympha tullia (Muller, 1764) (Lepidoptera: Satyrinae): habitat quality matters as much as patch size and isolation. J Insect Conserv 1:167–176. https://doi.org/10.1023/A:1018455714879
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Fan P, Ouyang Z, Nguyen DD, Nguyen TTH, Park H, Chen J (2019) Urbanization, economic development, environmental and social changes in transitional economies: Vietnam after Doimoi. Landsc Urban Plan 187:145–155. https://doi.org/10.1016/j.landurbplan.2018.10.014
Fleishman E, Betrus CJ, Blair RB, Nally RM, Murphy DD (2002) Nestedness analysis and conservation planning: the importance of place, environment, and life history across taxonomic groups. Oecologia 133:78–89. https://doi.org/10.1007/s00442-002-1003-8
Gaertner M, Larson BMH, Irlich UM, Holmes PM, Stafford L, van Wilgen BW, Richardson DM (2016) Managing invasive species in cities: A framework from Cape Town, South Africa. Landsc Urban Plan 151:1–9. https://doi.org/10.1016/j.landurbplan.2016.03.010
González-Oreja JA, De La Fuente-Díaz-Ordaz AA, Hernández-Santín L, Bonache-Regidor C, Buzo-Franco D (2012) Can human disturbance promote nestedness? Songbirds and noise in urban parks as a case study. Landsc Urban Plan 104:9–18. https://doi.org/10.1016/j.landurbplan.2011.09.001
González-Oreja JA, Zuria I, Carbó-Ramírez P, Charre GM (2018) Using variation partitioning techniques to quantify the effects of invasive alien species on native urban bird assemblages. Biol Invasions 20:2861–2874. https://doi.org/10.1007/s10530-018-1739-7
Gotelli NJ (2000) Null model analysis of species co-occurrence patterns. Ecology 81:2606–2621. https://doi.org/10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2
Habel JC, Samways MJ, Schmitt T (2019) Mitigating the precipitous decline of terrestrial European insects: Requirements for a new strategy. Biodivers Conserv 28:1343–1360. https://doi.org/10.1007/s10531-019-01741-8
Harini N (2002) Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl Geogr 22:175–186. https://doi.org/10.1016/S0143-6228(02)00002-4
Huang W (1993) Vegetation of Taiwan. China Environmental Science Press, Beijing, China
Jim CY, Chen WY (2008) Pattern and divergence of tree communities in Taipei’s main urban green spaces. Landsc Urban Plan 84:312–323. https://doi.org/10.1016/j.landurbplan.2007.09.001
Lee PF, Lin CC, Lo H, Ni MH, Yang SM, Chao WK (2016) Biodiversity of Taipei. Taipei City Animal Protection Office, Taipei, Taiwan
Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, MacIvor JS (2017) Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. Bioscience 67:799–807. https://doi.org/10.1093/biosci/bix079
MacArthur RH, Wilson EO (2001) The theory of island biogeography. Princeton University Press, Princeton, NJ
Moore JE, Swihart RK (2007) Toward ecologically explicit null models of nestedness. Oecologia 152:763–777. https://doi.org/10.1007/s00442-007-0696-0
Oliveira BF, Scheffers BR (2019) Vertical stratification influences global patterns of biodiversity. Ecography 42:249–249. https://doi.org/10.1111/ecog.03636
Parks and Street Lights Office (2018) Pass through the parks in Taipei City. https://parks.taipei/parks/index.php. Accessed 3 June 2021
Patterson BD, Atmar W (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linn Soc 28:65–82. https://doi.org/10.1111/j.1095-8312.1986.tb01749.x
Pinheiro RBP, Felix GMF, Dormann CF (2019) Mello MAR (2019) A new model explaining the origin of different topologies in interaction networks. Ecology 100(9):e02796. https://doi.org/10.1002/ecy.2796
Platt A, Lill A (2006) Composition and conservation value of bird assemblages of urban ‘habitat islands’: Do pedestrian traffic and landscape variables exert an influence? Urban Ecosyst 9:83–97. https://doi.org/10.1007/s11252-006-7900-6
Rico-Silva JF, Cruz-Trujillo EJ, Colorado ZGJ (2021) Influence of environmental factors on bird diversity in greenspaces in an Amazonian city. Urban Ecosyst 24:365–374. https://doi.org/10.1007/s11252-020-01042-x
Rouse JW Jr, Haas RH, Schell JA, Deering DW (1974) Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec Publ 351:309
Schouten MA, Verweij PA, Barendregt A, Kleukers RJM, de Ruiter PC (2007) Nested assemblages of Orthoptera species in the Netherlands: the importance of habitat features and life-history traits. J Biogeogr 34:1938–1946. https://doi.org/10.1111/j.1365-2699.2007.01742.x
Simberloff D, Cox J (1987) Consequences and Costs of Conservation Corridors. Conserv Biol 1:63–71
Simpson EH (1949) Measurement of Diversity. Nature 163:688–688
Tan X, Yang X, Chen C, Wang Y (2021) Nestedness and underlying processes of bird assemblages in Nanjing urban parks. Curr Zool 67:383–392. https://doi.org/10.1093/cz/zoaa069
Thebault E, Fontaine C (2010) Stability of Ecological Communities and the Architecture of Mutualistic and Trophic Networks. Science 329:853–856. https://doi.org/10.1126/science.1188321
Ulrich W (2009) Nestedness analysis as a tool to identify ecological gradients. Ecol Quest 11:27. https://doi.org/10.2478/v10090-009-0015-y
Ulrich W, Almeida-Neto M, Gotelli NJ (2009) A consumer’s guide to nestedness analysis. Oikos 118:3–17. https://doi.org/10.1111/j.1600-0706.2008.17053.x
Ulrich W, Gotelli NJ (2007) Null Model Analysis of Species Nestedness Patterns. Ecology 88:1824–1831. https://doi.org/10.1890/06-1208.1
United Nations, Department of Economic and Social Affairs, Population Division (2019) World urbanization prospects: The 2018 Revision (ST/ESA/SER.A/420). New York: United Nations
Vázquez DP, Melián CJ, Williams NM, Blüthgen N, Krasnov BR, Poulin R (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116:1120–1127. https://doi.org/10.1111/j.0030-1299.2007.15828.x
Wang X, Wang YP, Ding P (2012) Nested species subsets of amphibians and reptiles in Thousand Island Lake. Zool Res 33(5):439–446. https://doi.org/10.3724/SP.J.1141.2012.05439
Wang Y, Bao Y, Yu M, Xu G, Ding P (2010) Nestedness for different reasons: the distributions of birds, lizards and small mammals on islands of an inundated lake: Taxonomic differences in nestedness mechanisms. Divers Distrib 16:862–873. https://doi.org/10.1111/j.1472-4642.2010.00682.x
Zhang Y, Song T, Jin Q, Huang Y, Tang X, Sun X et al (2020) Status of an alien turtle in city park waters and its potential threats to local biodiversity: the red-eared slider in Beijing. Urban Ecosyst 23:147–157. https://doi.org/10.1007/s11252-019-00897-z
Acknowledgements
This study was funded by the Ministry of Science and Technology, Taiwan (Grant No. MOST 108-2621-M-002-010-MY3) for the funding support to this study. We sincerely thank the Taipei City Animal Protection Office for providing observation data of ecological surveys from 2007 to 2017.
Funding
This study was supported by the Ministry of Science and Technology, Taiwan (Grant No. MOST 108–2621-M-002–010-MY3).
Author information
Authors and Affiliations
Contributions
Material preparation and data analysis were performed by Rui-Qi Chen. Funding was acquired by Su-Ting Cheng. All authors contributed to the study conception and design, manuscript writing, and interpretation of the results.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interests.
Additional information
The original online version of this article was revised: there were 3 places required for corrections.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, RQ., Cheng, ST. Detecting nestedness in city parks for urban biodiversity conservation. Urban Ecosyst 25, 1839–1850 (2022). https://doi.org/10.1007/s11252-022-01272-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01272-1