Abstract
The aim of this study was to verify that Caragana korshinskii Kom. (CK) as a component of sheep forage influences lamb digestibility and rumen fermentation by altering the rumen microbial community. Hence, 12 female Tan sheep were allocated into 2 groups: receiving (CK group) or not (control group) 10% of the diet forage fraction with CK. During the 60-day experiment, growth performance, apparent digestibility, rumen volatile fatty acids (VFAs), and nitrogen balance were measured. Meanwhile, the rumen bacterial community diversity and composition were detected by the 16S rRNA sequence. The results indicated that the apparent digestibility of acid detergent fibre (ADF) tended to be higher (0.05 < P < 0.10), and the feed conversion efficiency was improved (P < 0.05) when CK was offered. Compared to those under alfalfa, the composition and abundance of the rumen microbial community were altered in the CK group, and the phylum Firmicutes, which is involved in promoting fibre digestion, increased in abundance. Moreover, VFAs tended to decrease (0.05 < P < 0.10), and the molar proportion of butyrate declined; similarly, levels of hypoxanthine and xanthine were lower (P < 0.05) in the sheep fed CK and may have been responsible for the decreased abundance of Fibrobacter spp., which are cellulolytic ruminal bacteria associated with VFA production.
Similar content being viewed by others
Introduction
Caragana korshinskii Kom. (CK) is widely grown in farming-pasture regions with dry-semidry climates in Northwestern China and is generally used as an afforestation shrub species for artificial vegetation restoration and desertification control owing to its ecological value (Long et al. 2020; Zeng et al. 2017). In contrast to its planting area, documented research on CK is scarce, and only a few papers have focused on this species, with plant-soil feedback, chemical component, and limited genetic studies in the literature. Occasionally, local herders use CK as forage in Northwestern China.
In recent years, CK has begun to receive attention as a forage bush, and it is known to have nutritional ingredients, being rich phenolic acids (gallic acid, syringic acid, tannins, etc.), alkaloids (hypaphorine), protein and mineral elements, and various essential and nonessential amino acids (Long et al. 2020; Luan et al. 2017; Zeng et al. 2017; Zhong et al. 2014). The total phenol content of CK reached 19.02 ± 0.57 mg/g (dry substance) by the alkaline extraction method (Zhong et al. 2014), while that of Medicago sativa L. (alfalfa), a commercialised legume with a short life cycle, is lower. Although phenolic acids, especially tannins, were known as antinutritional factors for a long time, studies in recent decades found that the suitable addition of tannins in fodder was beneficial for ruminants (Min et al. 2003). Tannins could reduce rumen nitrogen (N) digestibility, provide antiparasitic activity, and increase the yield of wool and lactoprotein and the ovulation rate in sheep (Min et al. 2003). The above results demonstrate that CK has tremendous potential as a feed forage. However, high amounts of CK (higher polyphenol) supplementation could lead to poor palatability and nutrient antagonism. Several studies reported that dietary polyphenol concentrations (over 2% dry matter, DM) had beneficial effects on sheep (Aerts et al. 1999), but a report indicated no positive relationship between polyphenol intake and growth performance, and there was no statistically significant difference when sheep were fed rosemary leaves as 10% and 20% of their diets (dietary polyphenols were 2000 and 4700 mg/kg DM, respectively) (Moñino et al. 2008). Therefore, CK as a feed additive for sheep should be comprehensively considered with regard to its palatability, nutrition, and the polyphenol contents of the feed.
In addition, rumen microorganisms play a key role in supplying nutrients and energy for ruminants, such as synthetising microbial true protein (MTP) and volatile fatty acids (VFAs) (Henderson et al. 2013), which ultimately are used to generate adipose tissue, the hyoideum, and lactoprotein. As diets with varying nutritional ingredients have an effect on rumen microorganisms (Zebeli et al. 2008), we speculate that supplementing feed with CK may affect N utilisation by influencing the microbial community of sheep.
This study aimed to assess the apparent digestibility, N balance, urinary purine derivatives, and rumen bacteria by partial supplementation of CK in sheep forage to compare nutrient metabolism and rumen bacterial communities between sheep receiving and not receiving CK.
Materials and methods
Animals and diets
Tan sheep were selected as the experimental animals, a Chinese indigenous breed belonging to the Mongolian series that is adapted to foraging in northern China. Twelve Tan sheep with similar initial body weight (BW, 27.50 ± 3.33 kg, 3 months of age, females) were purchased from the Ningxia livestock farm. The experimental animals were individually weighed and randomly divided into 2 groups (each with 6 lambs) and then housed in individual metabolic cages (height × length × width, 160 × 120 × 60 mm). The sheep were offered a diet containing 70% grass and 30% concentrate (Table 1 and S1) and fresh water ad libitum for the entire formal experiment. In control, the animals were fed a diet consisting of 70% alfalfa hay and 30% concentrate, and the sheep in the CK group received a diet composed of 60% alfalfa hay, 10% CK hay, and 30% concentrate. The concentrate contained 70% corn and 30% wheat middling and bran (DM basis).
All the experimental animals were adapted to the diets for 2 weeks (pre-trial period) and weighed before the 60-day feeding experiment (formal period) (Fig. S1). During both the pre-trial and formal periods, diets were provided twice a day at 7:00 and 17:00 and were offered ad libitum. At the end of the experiment, a 6-day digestibility trial was conducted between day 54 and day 59.
Sample collection and analysis
The sheep were weighed before the morning feeding at the beginning and the end of the formal period. Offered and refused feed amounts were recorded daily to calculate feed intake. From day 54 to 59, the apparent digestibility of the experimental diets was measured by using metabolic cages equipped with clean trays and tubs for the separate collection of faeces and urine. The diet samples were collected daily and mixed individually. Faeces were collected and weighed daily before the 7:00 feeding. For each sheep, 15% of the total daily excretion was collected in a plastic bag and stored at − 20 °C. Total daily urine was recorded and diluted with a sufficient 50% H2SO4 solution to keep the pH < 3. Ten millilitres of urine mix solution for each sheep was poured into a plastic tube daily and stored at − 20 °C. On day 59, the faecal or urine samples of each sheep were thoroughly blended, and 10% of the total samples were utilised for the chemical analyses (Zhou et al. 2015). The average daily feed intake (ADFI) was calculated following the removal and weighing of residual feed from the offered feed. Diets and faecal samples were sent to Ningxia Feed Engineering and Technology Research Centre for analysis of DM, organic matter (OM), crude protein (CP), ether extract (EE), neutral detergent fibre (NDF), acid detergent fibre (ADF), and N contents by following the China National or Professional Standards: GB/T 6435–2014, GB/T 6438–2007, GB/T 6432–2018, GB/T 6433–2006, NY/T 1459–2007, and GB/T 20,806–2006, respectively.
At the end of the experiment, on day 60, the ruminal fluid was collected before morning feeding and gathered using oral stomach tubes. Approximately 70 mL of the fluid was collected under a vacuum from each sheep. Digesta samples were measured for pH and immediately strained through four layers of cheesecloth. Afterwards, a 5 mL aliquot was mixed with the same volume of deproteinising solution (100 g metaphosphoric acid and 0.6 g crotonic acid/L) for the measurement of VFAs by using a gas chromatographer (Echrom A90E, Yi Meng Electronic Technology Co., Ltd., Shanghai, China) with a capillary column (AT-FFAP: 30.0 m × 0.32 µm × 0.33 µm, Lanzhou Institute of Chemical Physics, Lanzhou, China) (Zhou et al. 2015). After the 60-day trial, the sheep were slaughtered to determine the carcass characteristics. Warm carcass weight was measured within 30 min. The dressing rate was measured as the percentage ratio of the final body weight at slaughter and the warm carcass weight. The GR value was used to assess the fat content of the lamb carcasses and was measured as the total tissue thickness over the 12th rib 110 mm from the midline with a Vernier calliper.
The urine samples were also analysed for N content by Ningxia Feed Engineering and Technology Research Centre, and the values were used to calculate the N retention ratios. Urinary purine derivatives (PD) were determined using reverse-phase high-performance liquid chromatography (Agilent 1200, Agilent Technologies, Lexington, MA).
Total DNA of the ruminal fluid from each sheep was isolated using the TIANamp stool DNA kit (Tiangen, China). The V3–V4 regions of bacterial 16S rRNA were amplified by using the 341F/805R primer set (Herlemann et al. 2011). The PCR products were sequenced on an Illumina HiSeq 2500 platform (Health Genomics Bioinformatics Technology Co., Ltd., Beijing, China).
Bioinformatics and statistical analysis
Data of intake, digestibility, growth, ruminal fermentation, and carcass features were analysed by independent-samples T-test using SPSS 21 (IBM, Armonk, NY, USA). Differences were considered significant at P < 0.05, and trends were significant at 0.05 < P < 0.10.
The 16S amplicon sequencing data were quality filtered using FLASH. All sequence analyses were performed in QIIME 1.9.1 according to the QIIME instructions. Further error correction was performed using usearch61 with de novo models, and the remaining sequences were clustered into operational taxonomic units (OTUs) using UCLUST with 97% similarity. Taxonomic assignments of all OTUs were made using the Ribosomal Database Project (RDP) classifier within QIIME and the Greengenes13.8 reference database. Based on OTU tables, diversity indexes were calculated using alpha diversity and rank abundance scripts within the QIIME pipeline, and beta diversity was estimated based on Bray–Curtis distance and displayed by principal coordinate analysis (PCoA). The pheatmap and stats packages of the R4.0.3 were used to normalise and cluster the heatmap of genera (average linkage). Linear discriminant analysis effect size (LEfSe) was used to select the discrepant bacteria and their biological relevance between groups based on the nonparametric factor Kruskal–Wallis rank-sum test, Wilcoxon rank-sum test, and linear discriminant analysis.
Results
Growth performance and apparent digestibility
There was no significant difference in the initial and final BW, warm carcass weight, or ADFI between the 2 groups, while the feed-to-gain conversion ratio of the CK group was significantly lower than that of the control (P < 0.05). This result indicated that the addition of CK to sheep forage could result in a significantly better feed conversion efficiency (Table 2).
Table 3 lists the results of the apparent digestibility and N balance of the Tan sheep fed diets with and without CK. With the exception of EE, the other indices of apparent digestibility of DM, OM, CP, and NDF were higher in the CK group than in the control group (P > 0.10), and the digestibility of ADF tended to be significantly higher (P < 0.10). Even though the total N intake, faecal N excretion, and N retention were similar in the two groups (both P > 0.10), the urinary N excretion tended to be significantly smaller (0.05 < P < 0.10), while the ratio of N retention to N intake tended to be significantly higher in the CK group (0.05 < P < 0.10).
Rumen fermentation
Whether or not CK was added to the fodder, the ruminal pH was not affected (P > 0.10); only the concentration of total VFAs tended to be lower (0.05 < P < 0.10) when CK hay was substituted in the feed (Table 4), and no effects were observed for isobranch-chained VFAs between the two groups. Among the VFAs, the molar proportion of acetate was increased and butyrate was decreased significantly in the CK group (P < 0.05). Even though the difference in the proportion of propionate did not reach statistical significance, the ratio of acetate to propionate was increased significantly with the addition of CK hay (P < 0.05).
Urinary purine derivatives (PD) and PD nitrogen index (PNI)
The urinary PDs of the experimental sheep consisted mainly of allantoin (approximately 85%) and traces of uric acid, hypoxanthine, and xanthine (13–16% combined). From Table 5, urinary PDs excretion of hypoxanthine and xanthine were significantly lower in the diet containing CK (P < 0.05), whereas fractions of allantoin and hypoxanthine tended to be higher (0.05 < P < 0.10). Seemingly, PNI was similar for each group, and the total PD was 15.35 mmol/d in the control group versus 13.49 mmol/d in the CK group. The difference between the two groups was obvious but not significant.
Rumen bacterial community
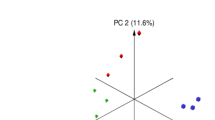
The alpha diversities of the microbiota between the control and treatment groups based on the Shannon diversity and Chao1 indexes are presented in Fig. 1a, with no significant differences. Samples displayed distinct clustering between the 2 groups in Fig. 1b, plotted by the Bray–Curtis distance (R = 0.2639, P = 0.007). According to 16S RNA sequencing, the dominant phyla in the rumen of the sheep either fed a diet with or without CK were Bacteroidetes, Firmicutes, and Proteobacteria at the phylum level, which accounted for 94% of the total bacterial relative abundance (Fig. 2a). When supplemented with CK, the composition of the bacterial community was altered, and the relative abundances of these three phyla accounted for 48.3%, 44.0%, and 2.3% in the treatment group and 51.6%, 39.8%, and 2.4% in the control, respectively. Although the average relative abundance of the phylum Bacteroidetes decreased 3.3% and Firmicutes increased 4.2% compared to the control group (P > 0.10), the difference was non-significant owing to individual variation. However, Verrucomicrobia (P = 0.039) and Fibrobacteres (P = 0.086) tended to significantly decrease in abundance compared with that in the control group. The abundance distribution of the top 50 genera between the 2 groups is displayed in a genus abundance heatmap, which revealed several genera that exhibited variations between groups (Fig. 2b). Based on the similarity within groups, it was apparent that the bacterial genera Akkermansia, Phascolarctobacterium, 5-7N15, Dorea, Bacteroides, rc4-4, Parabacteroides, BF311, Coprococcus, and Prevotella were more abundant in the ruminal microbiota of control group, while Mogibacterium, p-75-a5, and Selenomonas were more abundant in that of CK group.
Main rumen bacteria of Tan sheep offered diets with or without Caragana korshinskii Kom. a Bacterial alpha diversity based on Shannon diversity and Chao 1 indexes. b PCoA plot based on the Bray–Curtis dissimilarity of the microbiota. Each dot represents the composition of the microbiota of each sample. Samples were grouped by colour as the labels show. Control: diets without Caragana korshinskii Kom., n = 6; CK: diets with Caragana korshinskii Kom., n = 6
Comparisons of the rumen bacteria relative abundance in Tan sheep offered diets with or without Caragana korshinskii Kom. at different classification levels. a Bacterial taxonomic composition at the phylum level. The phyla Bacteroidetes and Firmicutes dominated the core microbiome in Tan sheep, followed by the phylum Proteobacteria. b A heatmap was generated from hierarchical clustering analysis of the normalised relative abundances of the top 50 genera in the 2 Tan sheep groups. Genera with square brackets are proposed by the Greengenes curators and indicate the recommended taxonomy. c Rumen bacteria with LDA scores greater than 2 were speculated to have different abundances between control and CK groups. d The cladogram displays the evolutionary relationship at three levels of the taxonomy (class, order, family) for taxa with scores over 2. Control: diets without Caragana korshinskii Kom., n = 6; CK: diets with Caragana korshinskii Kom., n = 6
The result of LEfSe showed that 5 and 3 taxa were detected as biomarkers (|LDA|> 2) in the control and treatment groups, respectively (Fig. 2c). Among these eight taxa with significant variation, family BS11, Rikenellaceae, Bacteroidaceae, genus 5_7N15, and Oscillospira decreased, while class MVP_15, order PL_11B10, and family RF16 increased under CK supplementation. Evolutionary relationship analysis showed that families BS11, Rikenellaceae, Bacteroidaceae, and RF16 belong to the order Bacteroidales, which had the highest relative abundance at the order level in both the control and treatment groups, and 5_7N15 was the main genus, with an approximately 50% abundance, in the family Bacteroidaceae. The decrease in abundance in family BS11, Rikenellaceae, and Bacteroidaceae was consistent with the tendency of the phylum Bacteroidetes to decrease in abundance, but the increase in RF16 was different. PL_11B10 was detected in only one control sample and in five treatment samples and is the unique order of class MVP_15 (Fig. 2d).
Discussion
Generally, Caragana korshinskii Kom. (CK) represented a higher ecological potential than Medicago sativa L. (alfalfa) for soil organic carbon, total nitrogen, and phosphorus sequestration in the soil–plant system (Fu et al. 2010), which resulted in widely growing in dry-semidry areas. Meanwhile, due to the strong regeneration ability, CK could be stumped at all seasons. Thus, CK partially replaces alfalfa in planting and sheep feeding would be appropriate from the standpoints of preserving the ecological environment and keeping the balanced nutrition. However, high levels of polyphenol in CK could result in poor palatability and finally affected feed intake, thus 10% of the diet forage fraction with CK hay was a maximum amount under ad libitum in our previous attempts. Most of the few pieces of research showed different forms and states of CK fed sheep, for instance, CK and concentrate were processed into pellet feeds would be conducive to CK intake up to 40% for sheep (Zhang et al. 2021). Or, 10% fermented CK in diet fed sheep could improve the intramuscular fat content (Xu et al. 2020). Furthermore, there is little report on the growth performance of other livestock, such as cattle, with feeding CK.
The two diets (without or with CK) were isonutrient formulated, the main nutrient components, such as OM, CP, EE, NDF, ADF, and gross energy (GE), were considered to be approximately equal in the two groups (Table 1). The experimental data for feeding, digestion, and metabolism showed an increase in the apparent digestibility of ADF, N retention to total N intake ratio, and a decrease in the feed conversion ratio. Less urinary N excretion implied that N utilisation efficiency of Tan sheep was improved with CK supplementation. These results indicated that partial substitution of alfalfa with CK did not lead to any adverse effects but had beneficial results in sheep feeding and digestion, although the contents of NDF and ADF were higher in CK than in alfalfa (Table S1). A study confirmed that when CK was used as an ingredient in the diets of sheep, the degradation rates of DM, NDF, ADF, and CP were up to 70%, 30%, 40%, and 80% at 24 h, respectively (Jiang et al. 2020). The results were consistent with the knowledge that CK is rich primarily in phenolic acids (Zeng et al. 2017), which are natural protein protectants to avoid rapid degradation by rumen microorganisms (Min et al. 2003).
Different to monogastric animals, rumen microbial true protein (MTP) is the main source of amino acids in ruminants (Zhou et al. 2016). Since MTP was proportional to purine derivative (PD) excretion in urine, urinary PD could be used as a practical indicator of MTP synthesis in sheep (Chen et al. 1995). A previous study showed that polyphenols could reduce the concentration of MTP by suppressing rumen bacterial enzyme activity, and this could be determined through the evaluation of urinary PDs (Jones et al. 1994). From our study, although no significant difference was observed between the two groups in total PD excretion, the total PD showed a decreasing tendency, and hypoxanthine and xanthine were lower in the CK supplement group (P < 0.05), accounting only for very small amounts of 6–9% combined, which might be in line with previous results. Previous research demonstrated that quebracho tannins had no influence on urinary PD excretion in sheep (Komolong et al. 2001). In any case, our results showed that PD excretion by sheep was closer to that of buffalo than to that previously reported in cattle (Cutrignelli et al. 2007). The PD nitrogen index (PNI) is another indicator that can estimate the efficiency of rumen degradable N conversion to MTP (Makkar and Chen 2004), and neither CK nor polyphenol affected the PNI. Studies have shown that plant polyphenols could inhibit xanthine oxidase in vitro, oxidising hypoxanthine and xanthine (Sabahi et al. 2018), which might have been the principal reason for hypoxanthine and xanthine reduction under CK supply in our study.
In this study, although the results showed that CK had no effect on alpha diversity, richness and evenness of the rumen microbiota, the samples grouped into 2 clusters significantly (P = 0.007) according to the Bray–Curtis distance metric (PCoA plot). Hence, feeding CK has a considerable influence on the microbiota composition of sheep. Many reports have proven that rumen microorganisms could be influenced by diet because different bacteria prefer different nutritional ingredients and rumen environments (Zebeli et al. 2008). Consistent with a previous study (Huang et al. 2017), the Bacteroidetes and Firmicutes phyla dominated the core microbiome in both groups of sheep; notwithstanding, the relative abundance of Proteobacteria in sheep that live in a hot environment may increase to become the third core phylum (Qian et al. 2017). Many bacteria in the phylum Firmicutes have the ability to decompose cellulose, and the higher relative abundance of Firmicutes in the CK group could promote fibre digestion and then improve the apparent digestibility of NDF and ADF because of its dominance in the rumen microbiota. Regarding other existing phyla that differed between the two groups, Verrucomicrobia was identified in 1997 and one of the bacterial phyla with aerobic methanotrophic capabilities. The reduction in Verrucomicrobia may have been a consequence of polyphenol changes affected by CK, similar to a study in which polyphenols were supplied in the diets of heifers (De Nardi et al. 2016). The phylum Fibrobacteres was reported to be critical for fibre degradation, and its members could be reduced by polyphenols (Bae et al. 1993), which was also in accordance with our results. Although both of these phyla affected the capacity for fibre degradation via polyphenols, their relative abundances were much lower than that of the high abundance phylum Firmicutes, and a few declining effects were counteracted.
In contrast to the higher relative abundance of the phylum Firmicutes, the genus Oscillospira (belonging to the phylum Firmicutes) was decreased in the CK group, and Oscillospira is a member of the family Ruminococcaceae, which includes many fibre-degrading bacteria and could be inhibited by polyphenols (McSweeney et al. 2001). An “out of sync” trend also appeared between the phylum Bacteroidetes and its family member RF16. Because of low abundance, the significant difference at the family level did not lead to the same difference trend at the phylum level. Moreover, there were the same growth rate trends between the phylum Bacteroidetes and its family members BS11, Bacteroidaceae, and Rikenellaceae, which can utilise a variety of substrates (Solden et al. 2017; Vibart et al. 2019).
To our knowledge, there have been no nutrition studies on the genus PL_11B10, as the unique order of class MVP_15 belonging to the phylum Spirochaetes, which includes many cellulolytic microbes that utilise a variety of substrates, such as cellulose, pectin and protein (Hess et al. 2011). According to environmental reports, the genus PL_11B10 could degrade organic matter in low-salinity petroleum reservoirs. On these grounds, CK as a feed additive may promote PL_11B10 growth to increase organisms with degradation abilities and aid host growth and metabolism.
According to a previous study (Zhong et al. 2014), the dietary polyphenol levels were approximately 1900 mg/kg (DM) in the CK group, which was similar to those achieved with polyphenol supplementation in sheep (Moñino et al. 2008). Some studies indicated that plant polyphenols could depress sheep or goat rumen fermentation by interacting with the bacterial cell wall or affecting ruminal microorganism enzymes ( Costa et al. 2018) and could be the most effective against cellulolytic bacteria by inhibiting Fibrobacter spp. (McSweeney et al. 2001), which belongs to the phylum Fibrobacteres. These microbes have been predicted to produce VFAs in the rumen (Hackmann et al. 2017). Therefore, a decrease in propionate and butyrate, as well as VFAs in the CK group, would be reasonable because of the inhibition of Fibrobacter spp. via the polyphenol-rich CK significantly decreased along with a decrease in the abundance of the phylum to which Fibrobacter belongs. Additionally, the content of NDF in CK was much higher than that in alfalfa, which was negatively correlated with VFA generation in vitro and in vivo (Njokweni et al. 2019), resulting in a reduction in VFAs in the CK group. The abundances of the VFA producers Akkermansia, Phascolarctobacterium, Dorea, Bacteroides, rc4-4, Parabacteroides, BF311, Coprococcus, and Prevotella (Bi et al. 2018; Feng et al. 2020; O’Hara et al. 2018) were reduced in the ruminal fluid under CK supplementation, still hinting that the reduction in VFAs was influenced by CK through decreasing VFA-producing bacteria.
For ruminants, VFAs provide energy for microbial fermentation and MTP synthesis, while propionic acid can provide more energy than acetic acid (Ben Shabat et al. 2016). Thus, the acetate to propionate ratio could influence the population structure of rumen microorganisms, affecting the nutrient and energy metabolism of the host (Ben Shabat et al. 2016; Sasson et al. 2017). Generally, a lower ratio of acetate to propionate is accompanied by an increase in N deposition for steers and sheep (Foiklang et al. 2016). Conversely, in our study, the higher acetate to propionate ratio of Tan sheep with CK supplementation than without CK was positively associated with N retention. These findings are difficult to explain with previous results and warrant further studies.
Conclusion
The current study indicated that partial (10%) replaced Medicago sativa L. (alfalfa) by Caragana korshinskii Kom. (CK) in diets seemed to have no influence on gaining weight but remarkable increase in feed conversion efficiency in sheep. Meanwhile, CK could increase the ADF degradation rate, N retention to total N intake ratio, and influence protein synthesis and VFA production through altered rumen bacterial structure, and highly increased the abundance of the phylum Firmicutes. This study preliminarily laid a foundation for using CK as a forage resource for ruminants and further researching CK polyphenol extracts as a feed additive.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aerts RJ, Barry TN, Mcnabb WC (1999) Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agriculture, Ecosystems & Environment 75:1-12. https://doi.org/10.1016/S0167-8809(99)00062-6
Bae HD, McAllister TA, Yanke J, Cheng KJ, Muir AD (1993) Effects of condensed tannins on endoglucanase activity and filter-paper digestion by fibrobacter-succinogenes S85. Applied and Environmental Microbiology 59 (7):2132-2138. https://doi.org/10.1128/aem.59.7.2132-2138.1993
Ben Shabat SK, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, Miller MEB, White BA, Shterzer N, Mizrahi I (2016) Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. The ISME journal 10 (12):2958-2972. https://doi.org/10.1038/ismej.2016.62
Bi Y, Zeng S, Zhang R, Diao Q, Tu Y (2018) Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiology 18 (1):69-69. https://doi.org/10.1186/s12866-018-1213-9
Chen XB, Mejia AT, Kyle DJ, Orskov ER (1995) Evaluation of the use of the purine derivative-creatinine ratio in spot urine and plasma samples as an index of microbial protein supply in ruminants - studies in sheep. Journal of Agricultural Science 125:137-143. https://doi.org/10.1017/s002185960007458x
Costa M, Alves SP, Cappucci A, Cook SR, Duarte A, Caldeira RM, McAllister TA, Bessa RJB (2018) Effects of condensed and hydrolyzable tannins on rumen metabolism with emphasis on the biohydrogenation of unsaturated fatty acids. Journal of Agricultural and Food Chemistry 66 (13):3367-3377. https://doi.org/10.1021/acs.jafc.7b04770
Cutrignelli MI, Piccolo G, D'urso S, Calabrò S, Bovera F, Tudisco R, Infascelli F (2007) Urinary excretion of purine derivatives in dry buffalo and Fresian cows, Italian Journal of Animal Science 6 (sup2): 563-566. https://doi.org/10.4081/ijas.2007.s2.563
De Nardi R, Marchesini G, Li S, Khafipour E, Plaizier KJ, Gianesella M, Ricci R, Andrighetto I, Segato S (2016) Metagenomic analysis of rumen microbial population in dairy heifers fed a high grain diet supplemented with dicarboxylic acids or polyphenols. BMC Veterinary Research 12:29. https://doi.org/10.1186/s12917-016-0653-4
Feng G, Mikkelsen D, Hoedt EC, Williams BA, Flanagan BM, Morrison M, Gidley MJ (2020) In vitro fermentation outcomes of arabinoxylan and galactoxyloglucan depend on fecal inoculum more than substrate chemistry. Food & Function 11 (9):7892-7904. https://doi.org/10.1039/d0fo01103g
Foiklang S, Wanapat M, Norrapoke T (2016) Effect of grape pomace powder, mangosteen peel powder and monensin on nutrient digestibility, rumen fermentation, nitrogen balance and microbial protein synthesis in dairy steers. Asian-Australasian Journal of Animal Sciences 29 (10):1416-1423. https://doi.org/10.5713/ajas.15.0689
Fu X, Shao M, Wei X, Horton R (2010) Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess Plateau of China. Geoderma 155(1-2):31-35. https://doi.org/10.1016/j.geoderma.2009.11.020
Hackmann TJ, Ngugi DK, Firkins JL, Tao J (2017) Genomes of rumen bacteria encode atypical pathways for fermenting hexoses to short-chain fatty acids. Environmental Microbiology 19 (11):4670-4683. https://doi.org/10.1111/1462-2920.13929
Henderson G, Cox F, Kittelmann S, Miri VH, Zethof M, Noel SJ, Waghorn GC, Janssen PH (2013) Effect of DNA extraction methods and sampling techniques on the apparent structure of cow and sheep rumen microbial communities. PloS One 8 (9):e74787. https://doi.org/10.1371/journal.pone.0074787
Herlemann DPR, Labrenz M, Juergens K, Bertilsson S, Waniek JJ, Andersson AF (2011) Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. The ISME journal 5 (10):1571-1579. https://doi.org/10.1038/ismej.2011.41
Hess M, Sczyrba A, Egan R, Kim T-W, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM (2011) Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331 (6016):463-467. https://doi.org/10.1126/science.1200387
Huang J, Li Y, Luo Y (2017) Bacterial community in the rumen of Tibetan sheep and Gansu alpine fine-wool sheep grazing on the Qinghai-Tibetan Plateau, China. The Journal of General and Applied Microbiology 63 (2):122-130. https://doi.org/10.2323/jgam.2016.08.003
Jiang B, Zhou Y, Wang T, Li F (2020) Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tan sheep. Bioengineered 11 (1):1159-1169. https://doi.org/10.1080/21655979.2020.1834740
Jones GA, McAllister TA, Muir AD, Cheng KJ (1994) Effects of Sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Applied and Environmental Microbiology 60 (4):1374–1378. https://doi.org/10.1128/aem.60.4.1374-1378.1994
Komolong MK, Barber DG, McNeill DM (2001) Post-ruminal protein supply and N retention of weaner sheep fed on a basal diet of lucerne hay (Medicago sativa) with increasing levels of quebracho tannins. Animal Feed Science & Technology 92 (1-2):59-72. https://doi.org/10.1016/s0377-8401(01)00246-2
Long Y, Liang F, Zhang J, Xue M, Zhang T, Pei X (2020) Identification of drought response genes by digital gene expression (DGE) analysis in Caragana korshinskii Kom. Gene 725.https://doi.org/10.1016/j.gene.2019.144170
Luan G, Tie F, Yuan Z, Li G, He J, Wang Z, Wang H (2017) Hypaphorine, an indole alkaloid isolated from Caragana korshinskii Kom., inhibites 3T3-L1 adipocyte differentiation and improves insulin sensitivity in vitro. Chemistry & Biodiversity 14 (7). https://doi.org/10.1002/cbdv.201700038
Makkar HPS, Chen XB (2004) Estimation of microbial protein supply in ruminants using urinary purine derivatives. Kluwer Academic Publishers, Dordrecht, Netherlands. https://doi.org/10.1007/978-1-4020-2844-1
McSweeney CS, Palmer B, Bunch R, Krause DO (2001) Effect of the tropical forage calliandra on microbial protein synthesis and ecology in the rumen. Journal of Applied Microbiology 90 (1):78-88. https://doi.org/10.1046/j.1365-2672.2001.01220.x
Min BR, Barry TN, Attwood GT, McNabb WC (2003) The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Animal Feed Science & Technology 106 (1-4):3-19. https://doi.org/10.1016/s0377-8401(03)00041-5
Moñino I, Martínez C, Sotomayor JA, Lafuente A, Jordán MJ (2008) Polyphenolic transmission to Segureno lamb meat from ewes' diet supplemented with the distillate from rosemary (Rosmarinus officinalis) leaves. Journal of Agricultural and Food Chemistry 56 (9):3363-3367. https://doi.org/10.1021/jf7036856
Njokweni SG, Weimer PJ, Warburg L, Botes M, van Zyl WH (2019) Valorisation of the invasive species, Prosopis juliflora, using the carboxylate platform to produce volatile fatty acids. Bioresource Technology 288.https://doi.org/10.1016/j.biortech.2019.121602
O'Hara E, Kelly A, McCabe MS, Kenny DA, Guan LL, Waters SM (2018) Effect of a butyrate-fortified milk replacer on gastrointestinal microbiota and products of fermentation in artificially reared dairy calves at weaning. Scientific Reports 8 (1):14901-14901. https://doi.org/10.1038/s41598-018-33122-6
Qian W, Li Z, Ao W, Zhao G, Wu J, Li G (2017) Bacterial community composition and fermentation in the rumen of Xinjiang brown cattle (Bos taurus), Tarim red deer (Cervus elaphus yarkandensis), and Karakul sheep (Ovis aries). Canadian Journal of Microbiology 63 (5):375-383. https://doi.org/10.1139/cjm-2016-0596
Sabahi Z, Farmani F, Soltani F, Moein M (2018) DNA protection, antioxidant and xanthine oxidase inhibition activities of polyphenol-enriched fraction of Berberis integerrima Bunge fruits. Iranian Journal of Basic Medical Sciences 21 (4):411–416. https://doi.org/10.22038/ijbms.2018.26563.6506
Sasson G, Ben-Shabat SK, Seroussi E, Doron-Faigenboim A, Shterzer N, Yaacoby S, Miller MEB, White BA, Halperin E, Mizrahi I (2017) Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow's capacity to harvest energy from its feed. mBio 8 (4). https://doi.org/10.1128/mBio.00703-17
Solden LM, Hoyt DW, Collins WB, Plank JE, Daly RA, Hildebrand E, Beavers TJ, Wolfe R, Nicora CD, Purvine SO, Carstensen M, Lipton MS, Spalinger DE, Firkins JL, Wolfe BA, Wrighton KC (2017) New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11. The ISME journal 11 (3):691-703. https://doi.org/10.1038/ismej.2016.150
Vibart RE, Ganesh S, Kirk MR, Kittelmann S, Leahy SC, Janssen PH, Pacheco D (2019) Temporal fermentation and microbial community dynamics in rumens of sheep grazing a ryegrass-based pasture offered either in the morning or in the afternoon. Animal 13 (10):2242-2251. https://doi.org/10.1017/s1751731119000168
Xu X, Liu T, Fan S, Ma W, Chen W, Zhang X (2020) Effects of fermented Caragana korshinskii on the intramuscular fat content and expression of FABP3, UBE3C, ADRB3, LIPE, and SCD in different muscles of Tan sheep. Czech Journal of Animal Science 65: 145–152. https://doi.org/10.17221/231/2019-CJAS
Zebeli Q, Tafaj M, Weber I, Steingass H, Drochner W (2008) Effects of dietary forage particle size and concentrate level on fermentation profile, in vitro degradation characteristics and concentration of liquid- or solid-associated bacterial mass in the rumen of dairy cows. Animal Feed Science & Technology 140 (3-4):307-325. https://doi.org/10.1016/j.anifeedsci.2007.04.002
Zeng Z, Ji Z, Hu N, Chen S, Bai B, Wang H, Suo Y (2017) Synchronous determination with double-wavelength by RP-HPLC-UV and optimization of ultrasound-assisted extraction of phenolic acids from Caragana species using response surface methodology. Journal of Pharmaceutical and Biomedical Analysis 140:182-189. https://doi.org/10.1016/j.jpba.2017.03.017
Zhang K, Qian Q, Mao Y, Xu Y, Yang Y, Chen Y, Wang X (2021) Characterization of growth phenotypes and gastrointestinal tract microbiota in sheep fed with caragana. Journal of Applied Microbiology 131(6): 2763–2779. https://doi.org/10.1111/jam.15138
Zhong C, Sun Z, Zhou Z, Jin M-J, Tan Z-L, Jia S-R (2014) Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. Journal of Agricultural and Food Chemistry 62 (14):3217-3222. https://doi.org/10.1021/jf500349s
Zhou J, Mi J, Degen A, Guo X, Wang H, Ding L, Qiu Q, Long R (2015) Apparent digestibility, rumen fermentation and nitrogen balance in Tibetan and fine-wool sheep offered forage-concentrate diets differing in nitrogen concentration. Journal of Agricultural Science 153 (6):1135-1145. https://doi.org/10.1017/S0021859615000465
Zhou Z, Bulgari O, Vailati-Riboni M, Trevisi E, Ballou MA, Cardoso FC, Luchini DN, Loor JJ (2016) Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. Journal of Dairy Science 99 (11):8956-8969. https://doi.org/10.3168/jds.2016-10986
Acknowledgements
We thank Dr. Yang Guo and doctoral candidates Wang Wenqiang and Ji Kaixi (Northwest Institute of Eco-Environment and Resources, CAS) for animal experiment assistance and Dr. Di Duolong (Lanzhou Institute of Chemical Physics, CAS) for assistance with chromatography analysis. This study was funded by the National Key R&D Programme of China (Grant No. 2016YFC0500709), the Strategic Priority Research Programme of the Chinese Academy of Sciences (Grant No. XDA26040305), and the Key Projects of the Chinese Academy of Sciences (Grant No. KFZD-SW-219).
Funding
This study was funded by the National Key R&D Programme of China (Grant No. 2016YFC0500709), the Strategic Priority Research Programme of the Chinese Academy of Sciences (Grant No. XDA26040305), and the Key Projects of the Chinese Academy of Sciences (Grant No. KFZD-SW-219).
Author information
Authors and Affiliations
Contributions
Methodology, software, data curation, and writing—original draft preparation, X.W.; investigation, X.H.; resources, Z.Z.; writing—review and editing and funding acquisition, Z.D.
Corresponding author
Ethics declarations
Ethics approval
Animal experiments were carried out at the experimental station of the Northwest Institute of Eco-Environment and Resources (NIEER), Chinese Academy of Sciences (CAS). All experiments were approved by NIEER, CAS (approval number: NIEER-190318–02).
Consent to participate
All the authors have read and approved the paper.
Consent for publication
All authors have agreed to the published manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Huang, X., Zhang, Z. et al. Effect of Caragana korshinskii Kom. as a partial substitution for sheep forage on intake, digestibility, growth, carcass features, and the rumen bacterial community. Trop Anim Health Prod 54, 190 (2022). https://doi.org/10.1007/s11250-022-03186-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03186-8