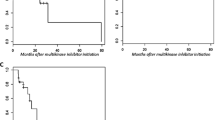
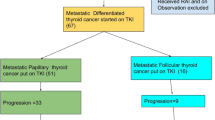
Abstract
Radioiodine (RAI) refractory differentiated thyroid cancer is an uncommon and challenging situation that requires a multidisciplinary approach to therapeutic strategies. The definition of RAI-refractoriness is usually a clear situation in specialized centers. However, the right moment for initiation of multikinase inhibitors (MKI), the time and availability for genomic testing, and the possibility of prescribing MKI and selective kinase inhibitors differ worldwide.
Latin America (LA) refers to the territories of the world that stretch across two regions: North America (including Central America and the Caribbean) and South America, containing 8.5% of the world’s population. In this manuscript, we critically review the current standard approach recommended for patients with RAI refractory differentiated thyroid cancer, emphasizing the challenges faced in LA. To achieve this objective, the Latin American Thyroid Society (LATS) convened a panel of experts from Brazil, Argentina, Chile, and Colombia. Access to MKI compounds continues to be a challenge in all LA countries. This is true not only for MKI but also for the new selective tyrosine kinase inhibitor, which will also require genomic testing, that is not widely available. Thus, as precision medicine advances, significant disparities will be made more evident, and despite efforts to improve coverage and reimbursement, molecular-based precision medicine remains inaccessible to most of the LA population. Efforts should be undertaken to alleviate the discrepancies between the current state-of-the-art care for RAI-refractory differentiated thyroid cancer and the present situation in Latin America.
Similar content being viewed by others
Abbreviations
- AE:
-
Adverse events
- ATA:
-
American Thyroid Association
- CR:
-
Complete response
- CT:
-
Computed tomography
- DTC:
-
Differentiated thyroid cancer
- ECOG:
-
Eastern Cooperative Oncologic Group
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- FDG-PET/CT:
-
18 F-fluorodeoxyglucose positron-emission tomography-computed tomography
- FTC:
-
Follicular thyroid cancer
- LA:
-
Latin America
- LATS:
-
Latin American Thyroid Society
- MAPK:
-
Mitogen-activated protein kinase
- MKI:
-
Multikinase inhibitors
- MRI:
-
Magnetic resonance imaging
- NGS:
-
Next-generation sequencing
- NIS:
-
Na/I symporter
- NTRK:
-
Neurotrophic tyrosine receptor kinase
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PI3K:
-
Phosphatidylinositol-3 kinase
- PR:
-
Partial response
- PTC:
-
Papillary thyroid cancer
- RAI:
-
Radioiodine
- RAI-R:
-
Radioiodine-refractory
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- RET:
-
Rearranged during transfection
- RTK:
-
Tyrosine kinase receptors
- SD:
-
Stable disease
- sTKI:
-
Selective tyrosine kinase inhibitor
- Tg:
-
Thyroglobulin
- TRKs:
-
Tropomyosin receptor kinase
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial Cancer. Int J Mol Sci 2021; 22:1726.
World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN: estimated cancer incidence, mortality and prevalence worldwide. Available from: http://globocan.iarc.fr.
Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2023: incidência do Câncer no Brasil. Rio de Janeiro: INCA, 2022. Available from: https://www.inca.gov.br/publicacoes/livros/estimativa-2023-incidencia-de-cancer-no-brasil.
Scheffel RS, Zanella AB, Antunes D, et al. Low recurrence rates in a cohort of differentiated thyroid carcinoma patients: a Referral Center Experience. Thyroid. 2015;25:883–9.
Ward LS, Scheffel RS, Hoff AO, et al. Treatment strategies for low-risk papillary thyroid carcinoma: a position statement from the thyroid Department of the brazilian society of Endocrinology and Metabolism (SBEM). Arch Endocrinol Metab. 2022;66:522–32.
Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9.
Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid Cancer: kinase inhibitors and Beyond. Endocr Rev. 2019;40:1573–604.
Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–8.
Fugazzola L, Elisei R, Fuhrer D et al. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur Thyroid J 2019; 8:227–245.
Tuttle RM, Ahuja S, Avram AM, et al. Controversies, Consensus, and collaboration in the Use of (131)I therapy in differentiated thyroid Cancer: a Joint Statement from the american thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the european thyroid Association. Thyroid. 2019;29:461–70.
Shonka DC Jr, Ho A, Chintakuntlawar AV, et al. American Head and Neck Society endocrine surgery section and international thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: defining advanced thyroid cancer and its targeted treatment. Head Neck. 2022;44:1277–300.
Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–90.
Romitti M, Ceolin L, Siqueira DR, et al. Signaling pathways in follicular cell-derived thyroid carcinomas (review). Int J Oncol. 2013;42:19–28.
Scheffel RS, de Cristo AP, Romitti M, et al. The BRAF(V600E) mutation analysis and risk stratification in papillary thyroid carcinoma. Arch Endocrinol Metab. 2021;64:751–7.
Giorgenon TMV, Carrijo FT, Arruda MA, et al. Preoperative detection of TERT promoter and BRAFV600E mutations in papillary thyroid carcinoma in high-risk thyroid nodules. Arch Endocrinol Metab. 2019;63:107–12.
Pessoa-Pereira D, Medeiros M, Lima VMS, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: a brazilian single-centre case series. Arch Endocrinol Metab. 2019;63:97–106.
Oler G, Cerutti JM. High prevalence of BRAF mutation in a brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115:972–80.
Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. 2022;34:9–18.
Yoo SK, Lee S, Kim SJ, et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12:e1006239.
Chu YH, Dias-Santagata D, Farahani AA, et al. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol. 2020;33:2186–97.
Galuppini F, Vianello F, Censi S, et al. Differentiated thyroid carcinoma in Pediatric Age: genetic and clinical scenario. Front Endocrinol (Lausanne). 2019;10:552.
Rangel-Pozzo A, Sisdelli L, Cordioli MIV et al. Genetic Landscape of Papillary thyroid Carcinoma and Nuclear Architecture: an overview comparing Pediatric and adult populations. Cancers (Basel) 2020;12:3146.
Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–107.
Pekova B, Sykorova V, Mastnikova K et al. NTRK Fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers (Basel) 2021;13:1932.
Ganly I, Ricarte Filho J, Eng S, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98:E962–972.
Penna GC, Pestana A, Cameselle JM, et al. TERTp mutation is associated with a shorter progression free survival in patients with aggressive histology subtypes of follicular-cell derived thyroid carcinoma. Endocrine. 2018;61:489–98.
Eszlinger M, Stewardson P, McIntyre JB et al. Systematic population-based identification of NTRK and RET fusion-positive thyroid cancers. Eur Thyroid J 2022;11:e210061.
Sciuto R, Romano L, Rea S, et al. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20:1728–35.
Pitoia F, Jauk F, Herzovich V, et al. Medicina de precisión en pacientes con carcinoma de tiroides derivado del epitelio folicular con fusiones del oncogén NTRK: la perspectiva argentina. Rev Argent Endocrinol Metab. 2022;59:7–16.
Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer. 2017;123:2955–64.
Haugen BR, Alexander EK, Bible KC et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26:1-133.
Kuba VM, Caetano R, Coeli CM, Vaisman M. Utility of positron emission tomography with fluorodeoxyglucose (FDG-PET) in the evaluation of thyroid cancer: a systematic review. Arq Bras Endocrinol Metabol. 2007;51:961–71.
Leboulleux S, Schroeder PR, Schlumberger M, Ladenson PW. The role of PET in follow-up of patients treated for differentiated epithelial thyroid cancers. Nat Clin Pract Endocrinol Metab. 2007;3:112–21.
Wang W, Larson SM, Tuttle RM, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001;11:1169–75.
Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505.
Esteva D, Muros MA, Llamas-Elvira JM, et al. Clinical and pathological factors related to 18F-FDG-PET positivity in the diagnosis of recurrence and/or metastasis in patients with differentiated thyroid cancer. Ann Surg Oncol. 2009;16:2006–13.
Rivera M, Ghossein RA, Schoder H, et al. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008;113:48–56.
Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–93.
Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 2011;21:1317–22.
American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, et al. Revised american thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214.
Jerkovich F, Abelleira E, Bueno F et al. Active Surveillance of Small Metastatic Lymph Nodes as an Alternative to Surgery in Selected Patients with Low-Risk Papillary Thyroid Cancer: A Retrospective Cohort Study. Thyroid 2022;32:1178–83.
Rondeau G, Fish S, Hann LE, et al. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011;21:845–53.
Tufano RP, Clayman G, Heller KS, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015;25:15–27.
Urken ML, Milas M, Randolph GW, et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the thyroid Cancer Care Collaborative. Head Neck. 2015;37:605–14.
Walter LB, Scheffel RS, Zanella AB et al. Active Surveillance of Differentiated Thyroid Cancer Metastatic Cervical Lymph Nodes: A Retrospective Single-Center Cohort Study. Thyroid 2023;33:312–20.
Nava CF, Scheffel RS, Cristo AP, et al. Neoadjuvant multikinase inhibitor in patients with locally advanced unresectable thyroid carcinoma. Front Endocrinol (Lausanne). 2019;10:712.
Danilovic DLS, Castro G Jr, Roitberg FSR, et al. Potential role of sorafenib as neoadjuvant therapy in unresectable papillary thyroid cancer. Arch Endocrinol Metab. 2018;62:370–5.
Wang JR, Zafereo ME, Dadu R, et al. Complete Surgical Resection following neoadjuvant Dabrafenib Plus Trametinib in BRAF(V600E)-Mutated anaplastic thyroid carcinoma. Thyroid. 2019;29:1036–43.
Kiess AP, Agrawal N, Brierley JD, et al. External-beam radiotherapy for differentiated thyroid cancer locoregional control: a statement of the american Head and Neck Society. Head Neck. 2016;38:493–8.
Nervo A, Ragni A, Retta F, et al. Interventional Radiology approaches for liver metastases from thyroid Cancer: a Case Series and Overview of the literature. J Gastrointest Cancer. 2021;52:823–32.
Wertenbroek MW, Links TP, Prins TR, et al. Radiofrequency ablation of hepatic metastases from thyroid carcinoma. Thyroid. 2008;18:1105–10.
Wexler JA. Approach to the thyroid cancer patient with bone metastases. J Clin Endocrinol Metab. 2011;96:2296–307.
Andrade F, Probstner D, Decnop M, et al. The impact of Zoledronic Acid and Radioactive Iodine Therapy on Morbi-Mortality of patients with bone metastases of thyroid Cancer derived from follicular cells. Eur Thyroid J. 2019;8:46–55.
Pitoia F, Jerkovich F, Trimboli P, Smulever A. New approaches for patients with advanced radioiodine-refractory thyroid cancer. World J Clin Oncol. 2022;13:9–27.
Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19:209–16.
Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28.
Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30.
Brose MS, Worden FP, Newbold KL, et al. Effect of age on the efficacy and safety of Lenvatinib in Radioiodine-Refractory differentiated thyroid Cancer in the Phase III SELECT Trial. J Clin Oncol. 2017;35:2692–9.
Tahara M, Kiyota N, Hoff AO, et al. Impact of lung metastases on overall survival in the phase 3 SELECT study of lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2021;147:51–7.
Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41(Suppl 2):1–S16.
Brose MS, Smit JWA, Lin CC, et al. Multikinase inhibitors for the treatment of asymptomatic Radioactive iodine-refractory differentiated thyroid Cancer: global noninterventional study (RIFTOS MKI). Thyroid. 2022;32:1059–68.
Filetti S, Durante C, Hartl DM, et al. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol. 2022;33:674–84.
Fullmer T, Cabanillas ME, Zafereo M. Novel therapeutics in Radioactive iodine-resistant thyroid Cancer. Front Endocrinol (Lausanne). 2021;12:720723.
Brose MS, Panaseykin Y, Konda B, et al. A randomized study of Lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid Cancer. J Clin Endocrinol Metab. 2022;107:776–87.
Brose MS, Robinson B, Sherman SI, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1126–38.
Jerkovich F, Califano I, Bueno F, et al. Real-life use of lenvatinib in patients with differentiated thyroid cancer: experience from Argentina. Endocrine. 2020;69:142–8.
Treistman N, Nobre GM, Tramontin MY, et al. Prognostic factors in patients with advanced differentiated thyroid cancer treated with multikinase inhibitors - a single brazilian center experience. Arch Endocrinol Metab. 2021;65:411–20.
Verburg FA, Amthauer H, Binse I, et al. Questions and controversies in the clinical application of tyrosine kinase inhibitors to treat patients with Radioiodine-Refractory differentiated thyroid carcinoma: Expert Perspectives. Horm Metab Res. 2021;53:149–60.
Haddad RI, Bischoff L, Ball D, et al. Thyroid carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:925–51.
Okamoto K, Ikemori-Kawada M, Jestel A, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6:89–94.
Gianoukakis AG, Dutcus CE, Batty N, et al. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer. 2018;25:699–704.
Laursen R, Wehland M, Kopp S, et al. Effects and Role of Multikinase inhibitors in thyroid Cancer. Curr Pharm Des. 2016;22:5915–26.
Wirth LJ, Brose MS, Elisei R et al. LIBRETTO-531: a phase III study of selpercatinib in multikinase inhibitor-naive RET-mutant medullary thyroid cancer. Future Oncol 2022.
Kim J, Bradford D, Larkins E, et al. FDA approval Summary: Pralsetinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27:5452–6.
Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9:491–501.
Chakravarty D, Johnson A, Sklar J, et al. Somatic genomic testing in patients with metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol. 2022;40:1231–58.
Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–40.
Waguespack SG, Drilon A, Lin JJ, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. 2022;186:631–43.
Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–82.
Busaidy NL, Konda B, Wei L, et al. Dabrafenib Versus Dabrafenib + Trametinib in BRAF-Mutated Radioactive Iodine Refractory differentiated thyroid Cancer: results of a Randomized, phase 2, open-label Multicenter Trial. Thyroid. 2022;32:1184–92.
Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:1272–82.
Alvarez-Gomez RM, De la Fuente-Hernandez MA, Herrera-Montalvo L, Hidalgo-Miranda A. Challenges of diagnostic genomics in Latin America. Curr Opin Genet Dev. 2021;66:101–9.
Araujo LH. Adopting Molecular Testing for Solid Tumors in Latin America: Challenges and Opportunities. Volume 3. RAS Oncology & Therapy; 2022.
Pitoia F, Smulever A, Jerkovich F. Letter to the Editor: “Foundation One Genomic Interrogation of Thyroid Cancers in Patients with Metastatic Disease Requiring Systemic Therapy”. J Clin Endocrinol Metab 2020; 105.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search and data analysis was made by all authors. The first draft of the manuscript was written by Fabian Pitoia and all authors commented on previous versions of the manuscript. Rafael Selbach Scheffel and Ana Luisa Maia critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
- Ana Luiza Maia: principal investigator in multicenter studies for Sanofi-Genzyme, Exelixis and Lilly.
- Fabian Pitoia: principal investigator in multicenter studies for Bayer, Exelixis and Novartis. Speaker for Bayer.
- Rafael Selbach Scheffel: subinvestigator in multicenter studies for Sanofi-Genzyme, Exelixis and Lilly.
- Ines Califano: subinvestigaton in multicenter studies for Novartis. Speaker for Bayer, Biotoscana/Knigtt, Raffo and Roche.
- Alicia Gauna: no conflicts of interest.
- Hernán Tala: no conflicts of interest.
- Fernanda Vaisman: principal investigator in multicenter studies for AstraZenca, Bayer, Lilly, Exelixis and Roche. Speaker for Abbott Laboratórios, Sanofi-Genzyme, Merck, Knight, Lilly, Ipsen, Onkos and Bayer. Advisory Board for Sanofi-Genzyme, Knight, Ipsen, Merck, Lilly and Bayer.
- Alejandro Roman Gonzalez: principal investigator in multicenter studies for Novartis, Sanofi and Amgen. Speaker for Biotoscana, Ultragenyx, Bayer, Ipsen, Amgen, Valentech and Recordati. Research fees for research from Corcept.
- Ana Oliveira Hoff: research support from Exelixis, Lilly and Novartis. Consulting (advisory board) for Exelixis, Knight, Eli Lilly and Bayer. Consulting (steering committee) for Eli Lilly. Speaker for Bayer.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pitoia, F., Scheffel, R.S., Califano, I. et al. Management of radioiodine refractory differentiated thyroid cancer: the Latin American perspective. Rev Endocr Metab Disord 25, 109–121 (2024). https://doi.org/10.1007/s11154-023-09818-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09818-0