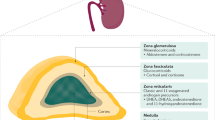
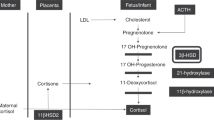
Abstract
Adrenal insufficiency requires prompt diagnosis in pregnancy, as untreated, it can lead to serious consequences such as adrenal crisis, intrauterine growth restriction and even foetal demise. Similarities between symptoms of adrenal insufficiency and those of normal pregnancy can complicate diagnosis. Previously diagnosed adrenal insufficiency needs monitoring and, often, adjustment of adrenal hormone replacement. Many physiological changes occur to the hypothalamic–pituitary–adrenal (HPA) axis during pregnancy, often making diagnosis and management of adrenal insufficiency challenging. Pregnancy is a state of sustained physiologic hypercortisolaemia; there are multiple contributing factors including high plasma concentrations of placental derived corticotropin-releasing hormone (CRH), adrenocorticotropin (ACTH) and increased adrenal responsiveness to ACTH. Despite increased circulating concentrations of CRH-binding protein (CRH-BP) and the major cortisol binding protein, corticosteroid binding globulin (CBG), free concentrations of both hormones are increased progressively in pregnancy. In addition, pregnancy leads to activation of the renin–angiotensin–aldosterone system. Most adrenocortical hormone diagnostic thresholds are not applicable or validated in pregnancy. The management of adrenal insufficiency also needs to reflect the physiologic changes of pregnancy, often requiring increased doses of glucocorticoid and at times mineralocorticoid replacement, especially in the last trimester. In this review, we describe pregnancy induced changes in adrenal function, the diagnosis and management of adrenal insufficiency in pregnancy and areas requiring further research.


Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AD:
-
Addison’s disease
- AI:
-
Adrenal insufficiency
- APS:
-
Autoimmune polyendocrine syndrome
- CAH:
-
Congenital adrenal hyperplasia
- CBG:
-
Corticosteroid binding globulin
- CRH:
-
Corticotropin releasing hormone
- CRH-BP:
-
Corticotropin releasing hormone binding protein
- HPA :
-
Hypothalamic–pituitary–adrenal
- NCCAH :
-
Non-classic congenital adrenal hyperplasia
- pCRH:
-
Placental corticotropin releasing hormone
- PNMT:
-
Phenylethanolamine N-methyltransferase
- PRA:
-
Plasma renin activity
- RAAS:
-
Renin-angiotensin-aldosterone system
- 11β-HSD1:
-
11 Beta hydroxysteroid dehydrogenase type 1
- 11β-HSD2:
-
11 Beta hydroxysteroid dehydrogenase type 2
- 11- DOC:
-
11 Deoxycorticosterone
References
Kamoun M, Mnif MF, Charfi N, et al. Adrenal diseases during pregnancy: pathophysiology, diagnosis and management strategies. Am J Med Sci. 2014;347(1):64–73. https://doi.org/10.1097/MAJ.0b013e31828aaeee.
Rushworth RL, Torpy DJ. Adrenal Insufficiency in Australia: Is it Possible that the Use of Lower Dose, Short-Acting Glucocorticoids has Increased the Risk of Adrenal Crises? Horm Metab Res. 2015;47(6):427–32. https://doi.org/10.1055/s-0034-1395680.
Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency Nat Rev Dis Primers. 2021;7(1):19. https://doi.org/10.1038/s41572-021-00252-7.
Cohen M. Addison’s disease complicated by toxemia of pregnancy; review of the literature. Arch Intern Med (Chic). 1948;81(6):879–87. https://doi.org/10.1001/archinte.1948.00220240088006.
Brent F. Addison’s disease and pregnancy. Am J Surg. 1950;79(5):645–52. https://doi.org/10.1016/0002-9610(50)90329-1.
Margulies SL, Corrigan K, Bathgate S, et al. Addison’s disease in pregnancy: Case report, management, and review of the literature. J Neonatal Perinatal Med. 2020;13(2):275–8. https://doi.org/10.3233/npm-190231.
Schneiderman M, Czuzoj-Shulman N, Spence AR, et al. Maternal and neonatal outcomes of pregnancies in women with Addison's disease: a population-based cohort study on 7.7 million births. BJOG. 2017;124(11):1772–9. https://doi.org/10.1111/1471-0528.14448.
Anand G, Beuschlein F. MANAGEMENT OF ENDOCRINE DISEASE: Fertility, pregnancy and lactation in women with adrenal insufficiency. Eur J Endocrinol. 2018;178(2):R45-r53. https://doi.org/10.1530/eje-17-0975.
Langlois F, Lim DST, Fleseriu M. Update on adrenal insufficiency: Diagnosis and management in pregnancy. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):184–92. https://doi.org/10.1097/med.0000000000000331.
Ambrosi B, Barbetta L, Morricone L. Diagnosis and management of Addison’s disease during pregnancy. J Endocrinol Invest. 2003;26(7):698–702. https://doi.org/10.1007/bf03347034.
Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev. 2005;26(6):775–99. https://doi.org/10.1210/er.2004-0025.
Gradden C, Lawrence D, Doyle PM, et al. Uses of error: Addison’s disease in pregnancy. Lancet. 2001;357(9263):1197. https://doi.org/10.1016/s0140-6736(05)71786-4.
Drucker D, Shumak S, Angel A. Schmidt’s syndrome presenting with intrauterine growth retardation and postpartum addisonian crisis. Am J Obstet Gynecol. 1984;149(2):229–30. https://doi.org/10.1016/0002-9378(84)90206-0.
O’Shaughnessy RW, Hackett KJ. Maternal Addison’s disease and fetal growth retardation. A case report J Reprod Med. 1984;29(10):752–6.
Bjornsdottir S, Cnattingius S, Brandt L, et al. Addison’s disease in women is a risk factor for an adverse pregnancy outcome. J Clin Endocrinol Metab. 2010;95(12):5249–57. https://doi.org/10.1210/jc.2010-0108.
Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–40. https://doi.org/10.1210/jc.2010-2395.
Magiakou MA, Mastorakos G, Webster E, et al. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Ann N Y Acad Sci. 1997;816:42–56. https://doi.org/10.1111/j.1749-6632.1997.tb52128.x.
Goland RS, Jozak S, Conwell I. Placental corticotropin-releasing hormone and the hypercortisolism of pregnancy. Am J Obstet Gynecol. 1994;171(5):1287–91. https://doi.org/10.1016/0002-9378(94)90149-x.
Goland RS, Wardlaw SL, Blum M, et al. Biologically active corticotropin-releasing hormone in maternal and fetal plasma during pregnancy. Am J Obstet Gynecol. 1988;159(4):884–90. https://doi.org/10.1016/s0002-9378(88)80162-5.
Linton EA, Perkins AV, Woods RJ, et al. Corticotropin releasing hormone-binding protein (CRH-BP): Plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76(1):260–2. https://doi.org/10.1210/jcem.76.1.8421097.
McLean M, Bisits A, Davies J, et al. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–3. https://doi.org/10.1038/nm0595-460.
Herrera CL, Bowman ME, McIntire DD, et al. Revisiting the placental clock: Early corticotrophin-releasing hormone rise in recurrent preterm birth. PLoS ONE. 2021;16(9): e0257422. https://doi.org/10.1371/journal.pone.0257422.
Magiakou MA, Mastorakos G, Rabin D, et al. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin Endocrinol (Oxf). 1996;44(4):419–28. https://doi.org/10.1046/j.1365-2265.1996.683505.x.
Demura R, Odagiri E, Yoshimura M, et al. Placental secretion of prolactin, ACTH and immunoreactive beta-endorphin during pregnancy. Acta Endocrinol (Copenh). 1982;100(1):114–9. https://doi.org/10.1530/acta.0.1000114.
Carr BR, Parker CR Jr, Madden JD, et al. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139(4):416–22. https://doi.org/10.1016/0002-9378(81)90318-5.
Demey-Ponsart E, Foidart JM, Sulon J, et al. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J Steroid Biochem. 1982;16(2):165–9. https://doi.org/10.1016/0022-4731(82)90163-7.
Nolten WE, Lindheimer MD, Oparil S, et al. Desoxycorticosterone in normal pregnancy. I. Sequential studies of the secretory patterns of desoxycorticosterone, aldosterone, and cortisol. Am J Obstet Gynecol. 1978;132(4):414–20.
Suri D, Moran J, Hibbard JU, et al. Assessment of adrenal reserve in pregnancy: Defining the normal response to the adrenocorticotropin stimulation test. J Clin Endocrinol Metab. 2006;91(10):3866–72. https://doi.org/10.1210/jc.2006-1049.
Ho JT, Lewis JG, O’Loughlin P, et al. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol (Oxf). 2007;66(6):869–77. https://doi.org/10.1111/j.1365-2265.2007.02826.x.
Avvakumov GV, Strel’chyonok OA. Evidence for the involvement of the transcortin carbohydrate moiety in the glycoprotein interaction with the plasma membrane of human placental syncytiotrophoblast. Biochim Biophys Acta. 1988;938(1):1–6. https://doi.org/10.1016/0005-2736(88)90115-0.
Avvakumov GV, Strel’chyonok OA. Properties and serum levels of pregnancy-associated variant of human transcortin. Biochim Biophys Acta. 1987;925(1):11–6. https://doi.org/10.1016/0304-4165(87)90142-5.
Abrao AL, Leal SC, Falcao DP. Salivary and serum cortisol levels, salivary alpha-amylase and unstimulated whole saliva flow rate in pregnant and non-pregnant. Rev Bras Ginecol Obstet. 2014;36(2):72–8.
Hodyl NA, Stark MJ, Meyer EJ, et al. High binding site occupancy of corticosteroid-binding globulin by progesterone increases fetal free cortisol concentrations. Eur J Obstet Gynecol Reprod Biol. 2020;251:129–35. https://doi.org/10.1016/j.ejogrb.2020.05.034.
Allolio B, Hoffmann J, Linton EA, et al. Diurnal salivary cortisol patterns during pregnancy and after delivery: Relationship to plasma corticotrophin-releasing-hormone. Clin Endocrinol (Oxf). 1990;33(2):279–89. https://doi.org/10.1111/j.1365-2265.1990.tb00492.x.
Abou-Samra AB, Pugeat M, Dechaud H, et al. Increased plasma concentration of N-terminal beta-lipotrophin and unbound cortisol during pregnancy. Clin Endocrinol (Oxf). 1984;20(2):221–8. https://doi.org/10.1111/j.1365-2265.1984.tb00077.x.
Nolten WE, Lindheimer MD, Rueckert PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab. 1980;51(3):466–72. https://doi.org/10.1210/jcem-51-3-466.
Odagiri E, Ishiwatari N, Abe Y, et al. Hypercortisolism and the resistance to dexamethasone suppression during gestation. Endocrinol Jpn. 1988;35(5):685–90. https://doi.org/10.1507/endocrj1954.35.685.
Magiakou MA, Mastorakos G, Rabin D, et al. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: Implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81(5):1912–7. https://doi.org/10.1210/jcem.81.5.8626857.
Robinson BG, Emanuel RL, Frim DM, et al. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988;85(14):5244–8. https://doi.org/10.1073/pnas.85.14.5244.
Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: Lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf). 1990;33(1):99–106. https://doi.org/10.1111/j.1365-2265.1990.tb00470.x.
Suda T, Iwashita M, Ushiyama T, et al. Responses to corticotropin-releasing hormone and its bound and free forms in pregnant and nonpregnant women. J Clin Endocrinol Metab. 1989;69(1):38–42. https://doi.org/10.1210/jcem-69-1-38.
Benediktsson R, Calder AA, Edwards CR, et al. Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf). 1997;46(2):161–6. https://doi.org/10.1046/j.1365-2265.1997.1230939.x.
Yang Q, Wang W, Liu C, et al. Compartmentalized localization of 11β-HSD 1 and 2 at the feto-maternal interface in the first trimester of human pregnancy. Placenta. 2016;46:63–71. https://doi.org/10.1016/j.placenta.2016.08.079.
Quinkler M, Oelkers W, Diederich S. Clinical implications of glucocorticoid metabolism by 11beta-hydroxysteroid dehydrogenases in target tissues. Eur J Endocrinol. 2001;144(2):87–97. https://doi.org/10.1530/eje.0.1440087.
Murphy BE. Conversion of cortisol to cortisone by the human uterus and its reversal in pregnancy. J Clin Endocrinol Metab. 1977;44(6):1214–7. https://doi.org/10.1210/jcem-44-6-1214.
Beitins IZ, Bayard F, Ances IG, et al. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7(5):509–19. https://doi.org/10.1203/00006450-197305000-00004.
Brue T, Amodru V, Castinetti F. MANAGEMENT OF ENDOCRINE DISEASE: Management of Cushing’s syndrome during pregnancy: Solved and unsolved questions. Eur J Endocrinol. 2018;178(6):R259–66. https://doi.org/10.1530/eje-17-1058.
Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R245–58. https://doi.org/10.1152/ajpregu.00108.2002.
Quinkler M, Meyer B, Bumke-Vogt C, et al. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol. 2002;146(6):789–99. https://doi.org/10.1530/eje.0.1460789.
Wilson M, Morganti AA, Zervoudakis I, et al. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am J Med. 1980;68(1):97–104. https://doi.org/10.1016/0002-9343(80)90178-3.
Ehrlich EN. Mineralocorticoids in normal and hypertensive pregnancies. Semin Perinatol. 1978;2(1):61–71.
Nolten WE, Lindheimer MD, Oparil S, et al. Desoxycorticosterone in normal pregnancy. II. Cortisol-dependent fluctuations in free plasma desoxycorticosterone. Am J Obstet Gynecol. 1979;133(6):644–8. https://doi.org/10.1016/0002-9378(79)90012-7.
Chanson P. Other Pituitary Conditions and Pregnancy. Endocrinol Metab Clin North Am. 2019;48(3):583–603. https://doi.org/10.1016/j.ecl.2019.05.005.
McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):Cd004454. https://doi.org/10.1002/14651858.CD004454.pub4.
Battarbee AN, Ros ST, Esplin MS, et al. Optimal timing of antenatal corticosteroid administration and preterm neonatal and early childhood outcomes. Am J Obstet Gynecol MFM. 2020;2(1): 100077. https://doi.org/10.1016/j.ajogmf.2019.100077.
Rushworth RL, Torpy DJ, Falhammar H. Adrenal Crisis. N Engl J Med. 2019;381(9):852–61. https://doi.org/10.1056/NEJMra1807486.
Bothou C, Anand G, Li D, et al. Current management and outcome of pregnancies in women with adrenal insufficiency: experience from a multicenter survey. J Clin Endocrinol Metab. 2020;105(8). https://doi.org/10.1210/clinem/dgaa266.
Yuen KC, Chong LE, Koch CA. Adrenal insufficiency in pregnancy: Challenging issues in diagnosis and management. Endocrine. 2013;44(2):283–92. https://doi.org/10.1007/s12020-013-9893-2.
Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–89. https://doi.org/10.1210/jc.2015-1710.
Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin Endocrinol (Oxf). 2013;78(4):497–502. https://doi.org/10.1111/cen.12097.
Reisch N. Pregnancy in Congenital Adrenal Hyperplasia. Endocrinol Metab Clin North Am. 2019;48(3):619–41. https://doi.org/10.1016/j.ecl.2019.05.011.
Bensing S, Giordano R, Falorni A. Fertility and pregnancy in women with primary adrenal insufficiency. Endocrine. 2020;70(2):211–7. https://doi.org/10.1007/s12020-020-02343-z.
Garelli S, Dalla Costa M, Sabbadin C, et al. Autoimmune polyendocrine syndrome type 1: an Italian survey on 158 patients. J Endocrinol Invest. 2021;44(11):2493–510. https://doi.org/10.1007/s40618-021-01585-6.
Husebye ES, Anderson MS, Kämpe O. Autoimmune Polyendocrine Syndromes. N Engl J Med. 2018;378(12):1132–41. https://doi.org/10.1056/NEJMra1713301.
Tallis PH, Rushworth RL, Torpy DJ, et al. Adrenal insufficiency due to bilateral adrenal metastases - A systematic review and meta-analysis. Heliyon. 2019;5(5): e01783. https://doi.org/10.1016/j.heliyon.2019.e01783.
Woodmansee WW. Pituitary Disorders in Pregnancy. Neurol Clin. 2019;37(1):63–83. https://doi.org/10.1016/j.ncl.2018.09.009.
Honegger J, Giese S. Acute pituitary disease in pregnancy: how to handle hypophysitis and Sheehan's syndrome. Minerva Endocrinol. 2018;43(4):465–75. https://doi.org/10.23736/s0391-1977.18.02814-6.
Falorni A, Laureti S, Nikoshkov A, et al. 21-hydroxylase autoantibodies in adult patients with endocrine autoimmune diseases are highly specific for Addison’s disease. Belgian Diabetes Registry Clin Exp Immunol. 1997;107(2):341–6. https://doi.org/10.1111/j.1365-2249.1997.262-ce1153.x.
Vila G, Fleseriu M. Fertility and pregnancy in women with hypopituitarism: A systematic literature review. J Clin Endocrinol Metab. 2020;105(3). https://doi.org/10.1210/clinem/dgz112.
Mercè Fernández-Balsells M, Muthusamy K, Smushkin G, et al. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia because of 21-hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin Endocrinol (Oxf). 2010;73(4):436–44. https://doi.org/10.1111/j.1365-2265.2010.03826.x.
Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–921. https://doi.org/10.1210/jc.2016-2118.
Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84(6):1997–2001. https://doi.org/10.1172/JCI114390.
Sidhu RK, Hawkins DF. Prescribing in pregnancy. Corticosteroids Clin Obstet Gynaecol. 1981;8(2):383–404.
Walsh SD, Clark FR. Pregnancy in patients on long-term corticosteroid therapy. Scott Med J. 1967;12(9):302–6. https://doi.org/10.1177/003693306701200902.
Stinson LJ, Stroud LR, Buka SL, et al. Prospective evaluation of associations between prenatal cortisol and adulthood coronary heart disease risk: The New England family study. Psychosom Med. 2015;77(3):237–45. https://doi.org/10.1097/PSY.0000000000000164.
Braun T, Challis JR, Newnham JP, et al. Early-life glucocorticoid exposure: The hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr Rev. 2013;34(6):885–916. https://doi.org/10.1210/er.2013-1012.
Reynolds RM. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38(1):1–11. https://doi.org/10.1016/j.psyneuen.2012.08.012.
Goedhart G, Vrijkotte TG, Roseboom TJ, et al. Maternal cortisol and offspring birthweight: Results from a large prospective cohort study. Psychoneuroendocrinology. 2010;35(5):644–52. https://doi.org/10.1016/j.psyneuen.2009.10.003.
Entringer S, Buss C, Andersen J, et al. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73(6):469–74. https://doi.org/10.1097/PSY.0b013e31821fbf9a.
Hohwü L, Henriksen TB, Grønborg TK, et al. Maternal salivary cortisol levels during pregnancy are positively associated with overweight children. Psychoneuroendocrinology. 2015;52:143–52. https://doi.org/10.1016/j.psyneuen.2014.11.006.
Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–62. https://doi.org/10.1016/s0140-6736(05)66617-2.
French NP, Hagan R, Evans SF, et al. Repeated antenatal corticosteroids: Effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190(3):588–95. https://doi.org/10.1016/j.ajog.2003.12.016.
Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol. 2014;10(7):391–402. https://doi.org/10.1038/nrendo.2014.73.
Huang W, Molitch ME. Pituitary Tumors in Pregnancy. Endocrinol Metab Clin North Am. 2019;48(3):569–81. https://doi.org/10.1016/j.ecl.2019.05.004.
Ost L, Wettrell G, Björkhem I, et al. Prednisolone excretion in human milk. J Pediatr. 1985;106(6):1008–11. https://doi.org/10.1016/s0022-3476(85)80259-6.
Ryu RJ, Easterling TR, Caritis SN, et al. Prednisone Pharmacokinetics During Pregnancy and Lactation. J Clin Pharmacol. 2018;58(9):1223–32. https://doi.org/10.1002/jcph.1122.
Scott EM, McGarrigle HH, Lachelin GC. The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. J Clin Endocrinol Metab. 1990;71(3):639–44. https://doi.org/10.1210/jcem-71-3-639.
Dörr HG, Heller A, Versmold HT, et al. Longitudinal study of progestins, mineralocorticoids, and glucocorticoids throughout human pregnancy. J Clin Endocrinol Metab. 1989;68(5):863–8. https://doi.org/10.1210/jcem-68-5-863.
Walker BR, Williamson PM, Brown MA, et al. 11 beta-Hydroxysteroid dehydrogenase and its inhibitors in hypertensive pregnancy. Hypertension. 1995;25(4 Pt 1):626–30. https://doi.org/10.1161/01.hyp.25.4.626.
Laatikainen T, Virtanen T, Kaaja R, et al. Corticotropin-releasing hormone in maternal and cord plasma in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1991;39(1):19–24. https://doi.org/10.1016/0028-2243(91)90136-9.
Kosicka K, Siemiątkowska A, Szpera-Goździewicz A, et al. Increased cortisol metabolism in women with pregnancy-related hypertension. Endocrine. 2018;61(1):125–33. https://doi.org/10.1007/s12020-018-1586-4.
Kosicka K, Siemiątkowska A, Krzyścin M, et al. Glucocorticoid metabolism in hypertensive disorders of pregnancy: Analysis of plasma and urinary cortisol and cortisone. PLoS ONE. 2015;10(12): e0144343. https://doi.org/10.1371/journal.pone.0144343.
Benassayag C, Souski I, Mignot TM, et al. Corticosteroid-binding globulin status at the fetomaternal interface during human term pregnancy. Biol Reprod. 2001;64(3):812–21. https://doi.org/10.1095/biolreprod64.3.812.
Mitchell E, Torpy DJ, Bagley CJ. Pregnancy-associated corticosteroid-binding globulin: High resolution separation of glycan isoforms. Horm Metab Res. 2004;36(6):357–9. https://doi.org/10.1055/s-2004-814580.
Strel’chyonok OA, Avvakumov GV. Interaction of human CBG with cell membranes. J Steroid Biochem Mol Biol. 1991;40(4–6):795–803. https://doi.org/10.1016/0960-0760(91)90305-o.
Bornstein SR, Breidert M, Ehrhart-Bornstein M, et al. Plasma catecholamines in patients with Addison’s disease. Clin Endocrinol (Oxf). 1995;42(2):215–8. https://doi.org/10.1111/j.1365-2265.1995.tb01866.x.
Rudman D, Moffitt SD, Fernhoff PM, et al. Epinephrine deficiency in hypocorticotropic hypopituitary children. J Clin Endocrinol Metab. 1981;53(4):722–9. https://doi.org/10.1210/jcem-53-4-722.
Morita S, Otsuki M, Izumi M, et al. Reduced epinephrine reserve in response to insulin-induced hypoglycemia in patients with pituitary adenoma. Eur J Endocrinol. 2007;157(3):265–70. https://doi.org/10.1530/eje-07-0176.
Merke DP, Chrousos GP, Eisenhofer G, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343(19):1362–8. https://doi.org/10.1056/nejm200011093431903.
Tutunculer F, Saka N, Arkaya SC, et al. Evaluation of adrenomedullary function in patients with congenital adrenal hyperplasia. Horm Res. 2009;72(6):331–6. https://doi.org/10.1159/000249160.
Verma S, Green-Golan L, VanRyzin C, et al. Adrenomedullary function in patients with nonclassic congenital adrenal hyperplasia. Horm Metab Res. 2010;42(8):607–12. https://doi.org/10.1055/s-0030-1253385.
Goldstein DS. Adrenaline and Noradrenaline, In: Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons, Ltd;2010.
Kim MS, Ryabets-Lienhard A, Bali B, et al. Decreased adrenomedullary function in infants with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99(8):E1597–601. https://doi.org/10.1210/jc.2014-1274.
Charmandari E, Weise M, Bornstein SR, et al. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: Potential clinical implications. J Clin Endocrinol Metab. 2002;87(5):2114–20. https://doi.org/10.1210/jcem.87.5.8456.
Zuckerman-Levin N, Tiosano D, Eisenhofer G, et al. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: A study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab. 2001;86(12):5920–4. https://doi.org/10.1210/jcem.86.12.8106.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise, in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Torpy, D.J. Adrenal insufficiency in pregnancy: Physiology, diagnosis, management and areas for future research. Rev Endocr Metab Disord 24, 57–69 (2023). https://doi.org/10.1007/s11154-022-09745-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09745-6