Abstract
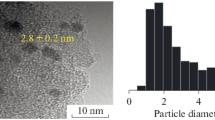
Nano-gold catalysts were prepared by the liquid reduction method and employed in the oxidative esterification of alcohols under ambient conditions. The effects of the supports and gold loading on the catalytic behavior were investigated. The catalysts were characterized by transmission electron microspectroscopy, X-ray diffraction, X-ray photoelectron microspectroscopy and atomic absorption spectrometry. The results indicated that nano-scale metallic state gold was highly dispersed on the support. 95 % methyl benzoate yield was obtained over 3 wt% Au/ZrO2 catalyst at 30 °C under air atmosphere. Under optimized reaction conditions, benzyl alcohols with electron-withdrawing groups could give high selectivity to the corresponding methyl ester at moderate conversion of alcohols. However, when a benzyl alcohol with electron-donating group was used, high conversion of alcohol but low selectivity of the corresponding methyl ester was obtained. Low yields were obtained with aliphatic alcohols as substrates even at high temperature. The kinetic analysis of the oxidation from benzyl alcohol to benzaldehyde and from benzaldehyde to methyl benzoate was also described. The facile and simple procedure was a great option for direct synthesis of methyl esters from alcohols.








Similar content being viewed by others
References
Corma A, Garcia H (2008) Chem Soc Rev 37:2096–2126
Guo H, Kemell M, Al-Hunaiti A, Rautiainen S, Leskelä M, Repo T (2011) Catal Commun 12:1260–1264
Choudhary VR, Dhar A, Jana P, Jha R, Uphade BS (2005) Green Chem 7:768–770
Otera J (1993) Chem Rev 93:1449–1470
Riehberg CE, Fisher CH (1944) J Am Chem Soc 66:1203–1207
Hiegel GA, Gilley CB (2003) Synth Commun 33:2003–2009
Karade NN, Tiwari GB, Huple DB (2005) Synlett 2005:2039–2042
Mori N, Togo H (2005) Tetrahedron 61:5915–5925
Pina CD, Falletta E, Rossi M (2012) Chem Soc Rev 41:350–369
Choudhary VR, Dumbre DK (2010) Appl Catal A Gen 375:252–257
Ma CY, Cheng J, Wang HL, Hu Q, Tian H, He C, Hao ZP (2010) Catal Today 158:246–251
Zahmakıran M, Özkar S (2010) Mater Chem Phys 121:359–363
Nielsen IS, Taarning E, Egeblad K, Madsen R, Christensen CH (2007) Catal Lett 116:35–40
Zheng N, Stucky GD (2007) Chem Commun 43:3862–3864
Liu G, Li G, Song H (2008) Catal Lett 128:493–501
Oliveira RL, Kiyohara PK, Rossi LM (2009) Green Chem 11:1366
Su F-Z, Ni J, Sun H, Cao Y, He H-Y, Fan K-N (2008) Chem Eur J 14:7131–7135
Parreira LA, Bogdanchikova N, Pestryakov A, Zepeda TA, Tuzovskaya I, Farías MH, Gusevskaya EV (2011) Appl Catal A Gen 397:145–152
Ishida T, Nagaoka M, Akita T, Haruta M (2008) Chem Eur J 14:8456–8460
Klitgaard SK, Riva AT, Helveg S, Werchmeister RM, Christensen CH (2008) Catal Lett 126:213–217
Miyamura H, Yasukawa T, Kobayashi S (2010) Green Chem 12:776
Hao Y, Chong Y, Li S, Yang H (2012) J Phys Chem C 116:6512–6519
Liu Z-P, Wang C-M, Fan K-N (2006) Angew Chem Int Ed 45:6865–6868
Manzoli M (2010) Appl Catal B 96:28–33
Zhang X, Shi H, Xu BQ (2011) J Catal 279:75–87
Cui W, Zhu H, Jia M (2013) Reac Kinet Mech Cat 109:551–562
Signoretto M (2013) Appl Catal B 129:287–293
Pinna F (2013) Catal Today 203:196–201
Zhu H, Ke X, Yang X, Sarina S, Liu H (2010) Angew Chem Int Ed 49:9657–9661
Liu C, Wang J, Meng L, Deng Y, Li Y, Lei A (2011) Angew Chem Int Ed 50:5144–5148
Gong J (2012) Chem Rev 112:2987–3054
Murdoch M, Waterhouse GIN, Nadeem MA, Metson JB, Keane MA, Howe RF, Llorca J, Idriss H (2011) Nat Chem 3:489–492
Musialska K, Finocchio E, Sobczak I, Busca G, Wojcieszak R, Gaigneaux E, Ziolek M (2010) Appl Catal A Gen 384:70–77
Primo A, Corma A, García H (2011) Phys Chem Chem Phys 13:595–602
Escamilla-Perea L, Nava R, Pawelec B, Rosmaninho MG, Peza-Ledesma CL, Fierro JLG (2010) Appl Catal A Gen 381:42–53
Abad A (2005) Angew Chem Int Ed 44:4066–4069
Yang J, Guan Y, Verhoeven T, van Santen R, Li C, Hensen EJM (2009) Green Chem 11:322–325
Acknowledgments
Financial supports from the National Natural Science Foundation of China, No. 20966008 and Opening Project of Natural Science Foundation of Inner Mongolia, No. 2010KF02 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, W., Jia, M., Ao, W. et al. Selective oxidative esterification of alcohols on Au/ZrO2 catalyst under ambient conditions. Reac Kinet Mech Cat 110, 437–448 (2013). https://doi.org/10.1007/s11144-013-0608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0608-8