Abstract
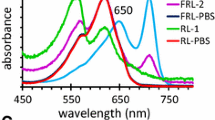
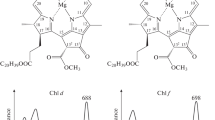
Far-red light photoacclimation (FaRLiP) is a mechanism that allows some cyanobacteria to utilize far-red light (FRL) for oxygenic photosynthesis. During FaRLiP, cyanobacteria remodel photosystem (PS) I, PS II, and phycobilisomes while synthesizing Chl d, Chl f, and far-red-absorbing phycobiliproteins, and these changes enable these organisms to use FRL for growth. In this study, a conjugation-based genetic system was developed for Synechococcus sp. PCC 7335. Three antibiotic cassettes were successfully used to generate knockout mutations in genes in Synechococcus sp. PCC 7335, which should allow up to three gene loci to be modified in one strain. This system was used to delete the rfpA, rfpB, and rfpC genes individually, and characterization of the mutants demonstrated that these genes control the expression of the FaRLiP gene cluster in Synechococcus sp. PCC 7335. The mutant strains exhibited some surprising differences from similar mutants in other FaRLiP strains. Notably, mutations in any of the three master transcription regulatory genes led to enhanced synthesis of phycocyanin and PS II. A time-course study showed that acclimation of the photosynthetic apparatus from that produced in white light to that produced in FRL occurs very slowly over a period 12–14 days in this strain and that it is associated with a substantial reduction (~34 %) in the chlorophyll a content of the cells. This study shows that there are differences in the detailed responses of cyanobacteria to growth in FRL in spite of the obvious similarities in the organization and regulation of the FaRLiP gene cluster.









Similar content being viewed by others
References
Airs RL, Temperton B, Sambles C, Farnham G, Skill SC, Llewellyn CA (2014) Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Lett 588:3770–3777
Akutsu S, Fujinuma D, Furukawa H, Watanabe T, Ohnishi-Kameyama M, Ono H, Ohkubo S, Miyashita H, Kobayashi M (2011) Pigment analysis of a chlorophyll f-containing cyanobacterium isolated from Lake Biwa. Photomed Photobiol 33:36–40
Allakhverdiev SI, Kreslavski VD, Zharmukhamedov SK, Voloshin RA, Korol’kova DV, Tomo T, Shen J-R (2016) Chlorophylls d and f and their role in primary photosynthetic processes of cyanobacteria. Biochemistry (Moscow) 81:201–212
Behrendt L, Brenjnrod A, Schliep M, Sørensen SJ, Larkum AW, Kühl M (2015) Chlorophyll f-driven photosynthesis in a cavernous cyanobacterium. ISME J 9:2108–2111
Brown II, Bryant DA, Casamatta D, Thomas-Keprta KL, Sarkisova SA, Shen G, Graham JE, Boyd ES, Peters JW, Garrison DH, McKay DS (2010) Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Appl Environ Microbiol 76:6664–6672
Bryant DA (1981) The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem 119:425–429
Bryant DA (1982) Phycoerythrocyanin and phycoerythrin: properties and occurrence in cyanobacteria. J Gen Microbiol 128:835–844
Bryant DA (1994) The molecular biology of cyanobacteria, advances in photosynthesis and respiration, vol 1. Kluwer, Dordrecht
Bryant DA, Cohen-Bazire G (1981) Effects of chromatic illumination on cyanobacterial phycobilisomes. Evidence for the specific induction of a second pair of phycocyanin subunits in Pseudanabaena 7409 grown in red light. Eur J Biochem 119:415–424
Chen M (2014) Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu Rev Biochem 83:317–340
Chen M, Schliep M, Willows RD, Cai Z-L, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329:1318–1319
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f—a red absorbing photopigment. FEBS Lett 586:3249–3254
Dubbs JM, Bryant DA (1991) Molecular cloning and transcriptional analysis of the cpeBA operon of the cyanobacterium Pseudanabaena species PCC7409. Mol Microbiol 5:3073–3085
Elhai J, Wolk CP (1988) Conjugal transfer of DNA to cyanobacteria. Meth Enzymol 167:747–754
Elhai J, Vepritskiy A, Muro-Pastor AM, Flores E, Wolk CP (1997) Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J Bacteriol 179:1998–2005
Gan F, Bryant DA (2015) Adaptive and acclimative responses of cyanobacteria to far-red light. Environ Microbiol 17:3450–3465
Gan F, Zhang S, Rockwell NC, Martin SS, Lagarias JC, Bryant DA (2014) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345:1312–1317
Gan F, Shen G, Bryant DA (2015) Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria. Life 5:4–24
Grotjohann I, Fromme P (2005) Structure of cyanobacterial photosystem I. Photosynth Res 85:51–72
Gutu A, Kehoe DM (2012) Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol Plant 5:1–13
Ho M-Y, Gan F, Shen G, Bryant DA (2016a) Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynth Res. doi:10.1007/s11120-016-0303-5
Ho M-Y, Shen G, Canniffe DP, Zhao C, Bryant DA (2016b) Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of Photosystem II. Science 353:aaf9178.
Kehoe DM, Gutu A (2006) Responding to color: the regulation of complementary chromattic adaptation. Annu Rev Plant Biol 57:127–150
Kühl M, Chen M, Ralph PJ, Schreiber U, Larkum AWD (2005) Ecology: a niche for cyanobacteria containing chlorophyll d. Nature 433:820
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris—a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5:67
Li Y, Lin Y, Garvey CJ, Birch D, Corkery RW, Loughlin PC, Scheer H, Willows RD, Chen M (2016) Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochim Biophys Acta 1857:107–114
Ludwig M, Bryant DA (2011) Transcription profiling of the model cyanobacterium Synechococcus sp. strain PCC 7002 by next-gen (SOLiD) sequencing of cDNA. Front Microbiol 2:41
Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S (1996) Chlorophyll d as a major pigment. Nature 383:402
Miyashita H, Ohkubo S, Komatsu H, Sorimachi Y, Fukayama D, Fujinuma D, Akutsu S, Kabayashi M (2014) Discovery of chlorophyll d in Acaryochloris marina and chlorophyll f in a unicellular cyanobacterium, strain KC1, isolated from Lake Biwa. J Phys Chem Biophys 4:149
Murakami A, Miyashita H, Iseki M, Adachi K, Mimuro M (2004) Chlorophyll d in an epiphytic cyanobacterium of red algae. Science 303:1633
Murray JW (2012) Sequence variation at the oxygen-evolving centre of photosystem II: a new class of ‘rogue’ cyanobacterial D1 proteins. Photosynth Res 110:177–184
Rippka R (1988) Isolation and purification of cyanobacteria. Meth Enzymol 167:3–27
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Shih PM, Wu D, Latifi A, Axen SD, Fewer DP, Talla E, Calteau A, Cai F, Tandeau de Marsac N, Rippka R, Herdman M, Sivonen K, Coursin T, Laurent T, Goodwin L, Nolan M, Davenport KW, Han CS, Rubin EM, Eisen JA, Woyke T, Gugger M, Kerfeld CA (2013) Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci USA 110:1053–1058
Sidler WA (1994) Phycobilisome and phycobiliprotein structure. In: Bryant DA (ed) Advances in photosynthesis and respiration, volume 1, the molecular biology of cyanobacteria. Kluwer Academic, Dordrecht, pp 139–216
Stanley DN, Raines CA, Kerfeld CA (2013) Comparative analysis of 126 cyanobacterial genomes reveals evidence of functional diversity among homologs of the redox-regulated CP12 protein. Plant Physiol 161:824–835
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Zhao Y, Shi Y, Zhao W, Huang X, Wang D, Brown N, Brand J, Zhao J (2005) CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that calcium ions regulate heterocyst differentiation. Proc Natl Acad Sci USA 102:5744–5748
Zhao C, Gan F, Shen G, Bryant DA (2015) RfpA RfpB and RfpC are the master control elements of far-red light photoacclimation (FaRLiP). Front Microbiol 6:1303
Acknowledgments
This research was supported by Grant MCB-1021725 from the National Science Foundation to D. A. B. This research was also conducted under the auspices of the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ho, MY., Gan, F., Shen, G. et al. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth Res 131, 173–186 (2017). https://doi.org/10.1007/s11120-016-0309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0309-z