Abstract
Aims
Nitrogen (N) addition effects on boreal forest ecosystem are influenced by an interplay between N-form and N-dose. We hypothesize that trees take up organic N more efficiently than inorganic N and that unwanted side-effects of organic N are smaller. We predicted that 1) the tree growth response to arginine (ARG) addition is larger than to ammonium-nitrate (AN) and, 2) understory vegetation and ectomycorrhizal (EcM) changes following ARG addition are smaller than following AN addition.
Methods
We investigated the effects of AN and ARG addition (50 and 150 kg N ha−1) during five years on tree growth, understory vegetation and EcM fungi in a Pinus sylvestris L. forest (c 50 years old) in northern Sweden.
Results
N addition increased tree growth and changed understory vegetation composition with few significant differences between AN and ARG. Differences in responses mainly occurred for the bryophyte Pleurozium schreberi which decreased more from ARG, and for EcM sporocarps, which sharply declined from AN, but not from ARG.
Conclusions
We found very few differences in responses between AN and ARG addition with the exception of EcM and bryophytes. These species groups have several key functions in boreal forests and the differences in responses merits further investigations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For more than a decade it has been known that conifer seedlings such as Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst) can be cultivated efficiently with nitrogen (N) supplied in the form of the amino acid arginine (ARG). (Öhlund and Näsholm 2001, 2002; Gruffman et al. 2012). These studies have documented lower leakage of N during nursery operations as well as enhanced root:shoot ratios and higher incidence of mycorrhizal root tips. It has also been demonstrated that root uptake rates of ARG is substantially higher than that of ammonium or nitrate (Öhlund and Näsholm 2001; Gruffman et al. 2013; Oyewole et al. 2016).
Organic N in the form of amino acids naturally dominates the soluble soil N pool directly available to plants and ectomycorrhizal (EcM) fungi in coniferous nutrient poor boreal forests (Jones and Kielland 2002; Kielland et al. 2007; McFarland et al. 2010; Inselsbacher and Näsholm 2012). Numerous short-term uptake studies have confirmed a high capacity of boreal forest plants (Näsholm et al. 1998; Nordin et al. 2001; Persson et al. 2003) as well as temperate trees (Scott and Rothstein 2011) to take up different amino acids. Ammonium is the main inorganic N form in boreal forest soils and many boreal plant species prefer ammonium over nitrate (Wallander et al. 1997; Marschner et al. 1991; Nordin et al. 2001). Nitrate uptake may even be inhibited by the addition of ammonium (Marschner et al. 1991; Gruffman et al. 2014), although some understory species, like the grass Avenella flexuosa (L.) Drejer, have a particularly high capacity for nitrate uptake (Nordin et al. 2006).
The N dose applied to a forest ecosystem may influence the distribution of the added N between different ecosystem compartments. Total ecosystem N recovery is higher at lower (≤50 kg N ha−1) than higher (>50 kg N ha−1) N doses, and a major proportion (c. 50%) of the added N is normally found in the soil (Melin and Nommik 1988; Templer et al. 2012). At high N doses (≥150 kg N ha−1) N may be lost through leakage (5–10% of the added N), mainly as nitrate which is mobile in the soil and there are concerns about eutrophication of receiving aquatic ecosystems (Binkley et al. 1999; Nohrstedt 2001; Saarsalmi and Mälkönen 2001). Plant N acquisition may increase disproportionally with increased N dose (Nordin et al. 1998; Nadelhoffer et al. 1999), although linear relations between increased plant growth and increased N dose have also been reported (Gundale et al. 2014; From et al. 2016).
There are numerous reports of effects on tree growth and species composition of the vegetation from additions of inorganic N. A single application of 150 kg N ha−1 in the form of ammoniumnitrate (AN) can enhance tree growth by 30% during a ten-year period (Nohrstedt 2001), while annually repeated AN applications may even double it (Bergh et al. 1999; Brockley 2010). A common N-addition effect on the boreal understory vegetation is transition to a species composition indicative of more fertile sites. For example grasses (e.g. A. flexuosa) and forbs are commonly promoted by AN addition, possibly due to their high capacity to take up N in the form of nitrate (Nordin et al. 2001, 2006; Persson et al. 2003), while more N-conservative ericaceous dwarf-shrubs efficiently take up organic forms or ammonium (e.g. Vaccinium spp.) may decrease (Nordin et al. 2001; Strengbom and Nordin 2008; Hedwall et al. 2013). Another common effect is a shift in species composition of bryophytes with a decrease in the most abundant species and in total cover (Strengbom et al. 2001; Nordin et al. 2009). These effects may be transient after a single AN application (Nohrstedt 2001), but may return in connection with disturbance of the tree layer (Strengbom and Nordin 2008, 2012).
Nitrogen addition to forest may also affect above- and belowground occurrence of ectomycorrhizal fungi (EcM). Most studies of EcM responses to N addition have been done in experiments with yearly N additions over several consecutive years or in naturally occurring gradients of inorganic N deposition. These studies indicate that responses to inorganic N addition are genus-specific. Genera like Cortinarius, Piloderma and Suillus have been reported to decline in abundance from long-term AN addition, while genera like Laccaria, Lactarius, Paxillus and Russula have been found to increase their sporocarp production (Lilleskov et al. 2001, 2002; Strengbom et al. 2001; Avis et al. 2003). More recently it has also been reported that organic N addition may enhance EcM colonization of tree seedlings growing in the field or in nurseries (Avolio et al. 2009; Gruffman et al. 2012).
Hence, it is clear that the forest ecosystem effects of N addition are influenced by an interplay between N form and N dose. The indications lately that organic N in the form of amino acids may be the dominant soluble N source directly available to uptake by plants in boreal forest (Inselsbacher and Näsholm 2012) may open up new solutions for increasing boreal forest productivity and carbon sequestration. We report the results of the first large-scale experiment investigating and comparing forest ecosystem effects of amino acid and AN addition in the field. We choose the amino acid arginine (ARG) as it is the most N-rich amino acid with an N-to-C ratio of 4:6. In the pH range normal for boreal forest soils it is positively charged and as such efficiently retained in the soil. We added ARG and AN to separate plots in two doses (50 and 150 kg N ha−1) to a c. 50-year-old Scots pine forest in northern Sweden. We hypothesized: 1) that the addition of ARG would result in a larger tree growth response than the addition of AN, and 2) that the addition of AN would cause relatively higher concentrations of mobile N in the soil and more pronounced species shifts in vegetation and fungal communities than the addition of ARG. We recorded basal area growth of trees, species composition of the forest floor vegetation and of above- and belowground EcM communities, and soil mobile N retained on ion exchange capsules for five years following the N addition.
Material and methods
Study site
The experiment was conducted in an even-aged (48 years) stand of Scots pine (P. sylvestris L.) with only a small number of other tree species; Picea abies (L.) Karst (0.2% of the basal area of trees) and Betula spp. (0.1%). The stand was planted at the site (64°11′N, 19°33′E) in 1964, 70 km from the Gulf of Bothnia in the province of Västerbotten, northern Sweden. The site had a flat topography and the soil was a podzol developed from glacial till with an organic layer of 15 cm. The ground vegetation was dominated by the ericaceous dwarf-shrub Vaccinium myrtillus L. with V. vitis-idaea L. and the grass A. flexuosa (L.) Drejer also being rather frequent. The bottom layer was dominated by the bryophyte Pleurozium schreberi (Brid.) Mitt. The background atmospheric N deposition for the area is estimated to be <2 kg N ha−1 yr.−1 and the mean annual temperature is 1 °C and mean annual precipitation is 600 mm (Swedish Meteorological and Hydrological Institute: www.smhi.se).
The experimental plots were distributed over an area of c. four hectares (ha). We set up 30 plots each sized 625 m2 (25 m × 25 m) with a 10 m buffer zone between adjacent plots in a complete randomized block design with five plots within each of the six blocks. The five treatments were untreated control, 50 and 150 kg N ha−1 as ARG and AN, and each treatment were replicated six times. A single application of 150 kg N ha−1 AN is used in practical forest fertilization in Scandinavia and the effects on the ecosystem are well-documented (Nohrstedt 2001) and thus suitable for a comparison with ARG. The lower dose was chosen to test if ARG could generate an increased tree growth at a lower dose than AN. Hence, there were two low-dose treatments (abbreviated to LARG and LAN) and two high-dose treatments (HARG and HAN). The powdery ARG and granulated AN was spread by hand in mid-June 2008 after the start of the vegetation period to promote uptake by trees and other vegetation.
Tree growth measurements
The diameter at breast height was measured on all trees in early autumn following the end of the growth season in 2008, 2011 and 2013. The growth response of Scots pine to N addition develops over a few years and mainly the immediate response is an increase of needle N concentrations as shoot growth is pre-determined already in the previous year. Thus, total basal area growth within each plot over the years 2009–2013 was used to assess treatment effects on tree growth in the statistical analyzes.
Soil mobile N estimated with ion exchange capsules
Ion exchange capsules (PST-1 resin capsule; UNIBEST International, Walla Walla, Washington, USA From et al. 2015) were inserted between the organic and mineral soil layers immediately before N addition in June 2008 to get an in situ estimate of mineralization of the added ARG and levels of ammonium and nitrate in the soil solution. These capsules were recovered in late October 2008 and new capsules were inserted. This was repeated so that capsules were kept in the soil from late May to October in 2009, from October 2009 to October 2010 and finally from October 2010 to October 2013. In each period, four to five capsules were put in each main plot. The capsules were extracted in 1 mol L−1 KCl, and the extracts were analyzed for concentrations of ammonium-N and nitrate-N with an AutoAnalyzer 3 (SEAL Analytical). Ammonium-N and nitrate-N per capsule (mg capsule−1) were calculated and the mean values of these within plot and sampling occasion were used as response variables.
Monitoring of sporocarps
Fungal sporocarps were counted every two weeks (six occasions) from mid-August to mid-October in 2008, 2009 and 2013. Two persons walked across each plot 7 times per person and counted sporocarps. To avoid double counting, counted sporocarps were cut off. Fungal species were identified mainly in situ. The sporocarps were summed over inventories within year and plot for further analyzes. The total number of sporocarps and the number of sporocarps from the four most common species/genera in the control treatment were used as response variables.
Identification of belowground ectomycorrhizal fungi
In mid to late August 2009, fine root systems of P. sylvestris in the humus layer were collected from five to seven locations in each plot. The roots from each location were put in plastic bags and kept in 4 °C for a maximum of two weeks before further analyses. The roots were washed carefully with tap water, and EcM root tips were assigned to morphotypes under a dissecting microscope. From each sampling location, a representative root tip from each morphotype was washed in an ultrasonicator and thereafter subjected to molecular identification. Extraction of DNA was performed using the CTAB method described by Ishida et al. (2007). The rDNA internal transcribed spacer (ITS) regions were amplified using ITS1F (Gardes and Bruns 1993) primer with a 5′ D2 fluorescent labeled (Sigma-Aldrich, France) and ITS4 primer (White et al. 1990) with a 5′ D3 fluorescent labeled. We also amplified the same ITS region using a 5′ D4 fluorescent labeled ITS1F and non-labeled ITS4 primers. For the polymerase chain reaction (PCR), KOD Plus DNA polymerase (Toyobo, Osaka, Japan) was mainly used; Qiagen Multiplex PCR kit was used when the product was faint or absent using KOD Plus DNA polymerase. We incubated 2 μl 5’ labeled PCR product with 1 U of either HinfI (Takara Shuzo, Shiga, Japan, or Fermentas, St. Leon-Rat, Germany) or TaqI (Takara Shuzo or Fermentas) in the reaction mixtures. The digested products and undigested both 5′ labeled PCR product were diluted with MilliQ water to standardize the fluorescent strength. The diluted products were added in 30 μl of formamide (Beckman Coulter, Fullerton, CA, USA) containing 0.31 μl of CEQ 600 size standard (Beckman Coulter). Capillary gel electrophoresis was performed using a CEQ 8000 (Beckman Coulter). The terminal restriction fragment lengths were determined using FRAGMENTS implemented in a CEQ 8800 genetic analysis system (Beckman Coulter). Four fragments (ITS1F with HinfI digested (labeled with D2), ITS4 with HinfI digested (D3), ITS1F with TaqI digested (D4) and undigested PCR product (D2 and D3)) were obtained for each sample, but small (<60 bp) and large (>700 bp) fragments were neglected due to the difficulty in determining the size. When samples had fragments within ±2 bp they were considered to be the same terminal restriction fragment length polymorphism (T-RFLP) type. Morphotype was also considered to determine the T-RFLP types.
The ITS region of each T-RFLP type was amplified again using non-labeled ITS1F and ITS4 primers, purified with a PCR product presequencing kit (USB Co., Cleveland, OH, USA), then its ITS sequence was determined by the commercially available DNA sequencing service (Eurofins MWG Operon, Ebersberg, Germany) or using Beckman 2000 (Beckman Coulter). Some T-RFLP types were sequenced using the samples from the EMF root tips (either on spruce or pine), or EMF sporocarps and Vaccinium spp. roots, which were collected in northern Sweden. When two or more sequences showed high identity (>98%) in ITS region (excluding the 18S and 28S rDNA region), these T-RFLP types were regarded as the same taxon. We mainly used ITS1F primer to determine the ITS sequence but ITS4 was used when necessary. The obtained sequences were compared with the sequences of known species in the UNITE database (Kõljalg et al. 2005) or in the GenBank by blast to infer the taxonomic position of the species. EMF morphotypes were assigned to T-RFLP types in each sampling location. The same T-RFLP types were pooled within plots and the relative abundance of each fungus was also calculated.
A total of 17,384 EcM root tips were morphotyped from 184 sampling locations 15 months following the N additions. Among 470 samples that were subjected to molecular identification, 410 samples were categorized as 49 T-RFLP types. ITS sequences were successfully determined for 40 T-RFLP types, in which six T-RFLP types were pooled to the other types due to the high sequence identity (>98%). The most dominant T-RFLP type (identified as Piloderma fallax) was observed in two morphotypes, each of which showed distinct color (yellow or silver) under the stereotype microscope. Because these morphotypes have different ITS sequences (data not shown), they were considered as different species in this study. Species of three T-RFLP types were determined according to the identified sporocarps that had matching T-RFs with root tips. Finally, 29 basidiomycota, 9 ascomycota and 6 unknown species were obtained and regarded as putative species in this study (ESM 1). Among the remaining 60 samples, 44 were assigned to a known species based on their morphological characteristics (Cenococcum geophilum or Piloderma fallax (yellow type); ESM 1). T-RFLP types of the remaining 16 samples were unknown, corresponding to 3.7% of the examined root tips, and were removed from further analyses.
Monitoring of forest floor vegetation
Forest floor vegetation was monitored in seven permanent subplots in each main plot. We used the point-intercept method with a frame sized 0.2 × 0.6 m with 30 random points. At each point a stick (4 mm diameter) was inserted to the vegetation and the number of hits of individual plant species was counted. Monitoring was conducted in August 2007 (a year before the N addition treatments), in August each year 2008, 2009 and 2013. The number of hits per m2 was calculated and the mean values within plot and year for the five most common species/species groups were used as response variables.
Statistical analyzes
All statistical analyzes were done in R 3.1.1 (R Core Team 2014). Generalized Linear Mixed Models (GLMM) with block as a random variable, and treatment and year/sampling occasion as fixed factors, were used to model the effects of N addition and time, as well as the interaction between N addition and time. When a significant treatment effect was identified in a single year, the GLMM was followed by a Generalized Linear Models (GLM) for that certain year with block as a fixed factor. If the block-effect was non-significant the block factor was removed from the models. Additionally, a factorial variable, indicating the location of a plot in relation to the stand border, was included in the full models of belowground EMF. The GLMs was followed by planned contrasts of the following pairs of treatments; LAN-LARG, HAN-HARG, LAN-HAN, LARG-HARG, with a correction of the P-values by the fdr-method (Benjamini and Hochberg 1995). All response variables were modelled with a Gamma error distribution and log-link. The models were evaluated and checked for heteroscedasticity and other remaining patterns in the residuals by plotting the residuals against the predicted values. A threshold of P = 0.05 was used to define significant differences between treatments. The GLMMs were done by applying the glmmPQL function in the MASS package (Venables and Ripley 2002) while the pair-wise comparisons were done by the glht function in the multcomp package (Hothorn and Westfall 2008).
Results
Tree growth
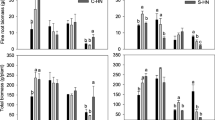
During the five-year period following the N additions the stand basal area had increased by 2.3 m2 ha−1 in the control treatment. The basal area growth was significantly higher in three of the N addition treatments (LAN, HAN and HARG) than in the control (Fig. 1). The highest growth was observed in the high AN treatment (HAN) (+40%), but there were no significant differences between the three different N treatments showing a significant growth response (LAN, HAN and HARG) (Fig. 1).
The basal area (BA) change between 2009 and 2013 in the five treatments: LAN = 50 kg N ha−1 AN, LARG = 50 kg N ha−1 ARG, HAN = 150 kg N ha−1 AN, HARG = 150 kg N ha−1 ARG. Treatments with rastered bars were significantly different from the control (P < 0.05) in the GLM. There were no significant differences in basal area growth between the three significant N addition treatments according to the planned contrasts analysis
Mobile soil N retained on ion capsules
Nitrogen addition caused, independently of N-form or dose, elevated amounts of ammonium retained on the ion exchange capsules during the growth season in the year of N addition (2008) (Fig. 2). The highest amount of ammonium was observed in the HAN treatment with more than 90 times the amount retained on the capsules than in the control (Fig. 2). There was a significant effect of N-form on ammonium-N in the low-dose treatments. According to the planned contrasts low addition of AN (LAN) caused significantly larger (P = 0.004) amounts of ammonium-N on the capsules than low addition of ARG (LARG) (Fig. 2). There was no significant difference between the doses of AN (P = 0.091) but the capsules in HARG retained significantly larger amounts of ammonium-N than the ones in LARG (P < 0.001) (Fig. 2).
The amounts of ammonium-N and nitrate-N retained on ion exchange capsules during the growth season in the year of the N addition in the five treatments. Treatments with rastered bars were significantly different from the control (P < 0.05) in the GLMM. Bars with different letters indicate significant pair-wise contrasts based on a GLM (P < 0.05). Observe that the following contrasts were included in the analyzes: LAN-LARG, HAN-HARG, LAN-HAN and LARG-HARG
The GLMM also showed elevated amounts of nitrate-N retained on the capsules in LAN (P < 0.001) and HAN (P < 0.001) compared to the control treatment (Fig. 2). Significant contrasts were found between N-forms within dose in which capsules in LAN retained larger amounts than LARG (P < 0.001) and HAN higher than HARG (P < 0.001), while there was no significant difference between LAN and HAN, and between LARG and HARG (Fig. 2). From the winter 2008/2009 and on, both the amounts of ammonium-N and of nitrate-N returned to levels equal to those on control plots and there were no further significant effects of any of the N addition treatments (data not shown).
Production of fungal sporocarps
The total number of fungal sporocarps was significantly lower in plots treated with AN than in the control plots (Fig. 3) while there was no such effect of ARG. This effect was, however, only discernable in 2009, the year following the N addition, but this year also had a generally higher abundance of sporocarps than the other studied years (P = 0.027). Significant contrasts between treatments in 2009 were found between HAN and HARG (P < 0.001) and close to significant between LAN and LARG (P = 0.057), while there were no differences between the doses within N-form (Fig. 3).
Number of sporocarps found in 2009 divided on the four most abundant species/species groups in the five treatments. Combinations of species and treatments with rastered bars were significantly different from the control (P < 0.05) in the GLMM. Parts of bars with different letters indicate significant pair-wise contrasts based on a GLM (P < 0.05). Observe that the following contrasts were included in the analyzes: LAN-LARG, HAN-HARG, LAN-HAN and LARG-HARG
Both C. semisanguinus and Cortinarius spp. had lower number of sporocarps in LAN, HAN and HARG than in the control in 2009. On the other hand, there was no significant difference between the control and LARG (Fig. 3) indicating an effect of dose in ARG with larger effects at the higher dose, something that was absent in the AN treatments. There were significant contrasts between LAN and LARG (P = 0.043), HAN and HARG (P < 0.001) and between LAN and HAN (P < 0.001) in number of C. semisanguinus sporocarps (Fig. 3). Significant differences between the control and LAN, and the control and HARG, were found for the number of Laccaria bicolor and Lactaria rufus sporocarps, respectively. Both these species were significantly more abundant in the HARG than in the LARG and HAN treatments. Hence, although there was no significant effect of ARG on the total number of sporocarps, there was a considerable shift in species dominance in the ARG treatment with the highest dose (HARG) (Fig. 3).
Belowground EcM
Root tip EcM community was dominated by C. geophilum (in average over all plots on 21% of the root tips) and three Piloderma species (15, 15 and 11% of the root tips, respectively). All the other species were found on less than 3% of the root tips and observed on less than 13 of the plots. Among these four dominant species C. geophilum was found on a significantly higher share of the root tips in HAN than in the control according to the GLM (P = 0.045) and a higher share in LAN than in HAN in the planned contrasts (P = 0.014). Additionally, there was a larger share of Piloderma sp. in LAN than in the control (P = 0.002). A complete species list can be found in ESM 1.
Species composition of forest floor vegetation
There were no pre-N-addition differences between the treatments in abundance of any of the analyzed ground vegetation species (Fig. 4). Already in late summer 2008 (two months after N addition), the abundance of the narrow-leaved grass A. flexuosa had responded with an increased abundance in all N addition treatments (Fig. 4). The general increase in this species due to fertilization remained in 2009 and 2013, although with a smaller effect in 2013 (Fig. 4). There were no effects of N-form on A. flexuosa in any of the years but significant differences between the AN doses in 2009 (P = 0.039) and between the ARG doses in 2008 (P = 0.003), 2009 (P = 0.039) and 2013 (P = 0.016) (Fig. 4). There were no effects of N addition on the abundance of V. myrtillus or forbs in any of the analyzed years (Fig. 4). Similarly, V. vitis-idaea did not show any response to N addition during the two first inventories. In 2013, however, the abundance of this species was significantly lower in all N treatments than in the control, while none of the analyzed contrasts were significant. The abundance of the bottom layer bryophyte Pleurozium schreberi was significantly lower in all N treatments other than LAN in 2009, and in all N treatments in 2013. HARG had a lower (P = 0.001–0.040) abundance of this bryophyte than LARG and LAN in both these years (Fig. 4) suggesting an effect of N-form and dose.
The abundance (hits per plot) of five species/species groups in the forest floor vegetation in 2007 (before N addition), in August 2008 (two months after N addition), and in August of 2009 and 2013 in plots of the five treatments. Treatments with rastered bars were significantly different from the control (P < 0.05) in the GLMM. Bars with different letters indicate significant pair-wise contrasts based on a GLM (P < 0.05). Observe that the following contrasts were included in the analyzes: LAN-LARG, HAN-HARG, LAN-HAN and LARG-HARG
Discussion
In this field study the plot-scale application of ARG and AN resulted in significantly increased basal area growth compared to the control. However, the stimulation of growth was similar for the two N sources. Hence our data do not support the hypothesis that ARG addition would result in a greater tree growth response than AN addition. We also predicted that unwanted side effects of N addition on ground vegetation and mycorrhizal fungi would be lesser from ARG than AN addition. We found that effects differed between the N forms mainly as the bryophyte Pleurozium schreberi decreased more from ARG, and as EcM sporocarps declined from AN, but not from ARG.
Our investigation of mycorrhizal colonization of pine root tips one year following the N addition, showed no effect on the overall root tip colonization from neither of the N forms. The responses of ectomycorrhizas to N addition are known to be variable. Some studies report no changes in coniferous forests (Fransson et al. 2000; Peter et al. 2001; Berch et al. 2009; Hay et al. 2015), while others report significant reductions in abundance and changes in species composition (Lilleskov et al. 2002; Frey et al. 2004; Berch et al. 2006; Jones et al. 2012). The N addition effects on ectomycorrhizal sporocarp production were, however, more pronounced. The overall abundance of sporopcarps on AN plots was less than half of that on ARG or control plots. Mainly it was sporocarps of Cortinarius spp. that declined from AN additions. There was a significant dose effect as the high AN dose caused a significantly larger decline of sporocarps than the low AN dose. Also the high ARG dose caused a significant decline of sporocarps in comparison to control plots, while the low ARG dose had no such effect. Negative effects of inorganic N addition on Cortinarius spp. sporocarp production have previously been reported from experiments with annually repeated N additions, and may last for as much as 50 years after the termination of N additions (Strengbom et al. 2001; Hasselquist and Högberg 2014). Noticeable was also the strong increase of Lactarius rufus (Scop:Fr) Fr sporocarps on high ARG plots. The proportion of this species of the total species pool was more than doubled on high ARG plots compared to low ARG plots and control plots. Other studies have shown that inorganic N addition may promote both tree root colonization and sporocarp production of Lactarius spp. (Strengbom et al. 2001; Hasselquist and Högberg 2014; Suz et al. 2015) and our result corroborate L. rufus’ capacity to proliferate under circumstances of elevated N supply, although only when N was supplied in an organic form.
Nitrogen addition effects on boreal forest floor vegetation have been extensively investigated mainly in experiments with repeated N additions (see for example Nohrstedt 2001 and references therein; Nordin et al. 2009; Hedwall et al. 2013; Manninen and Tolvanen 2013). Here we show that the proliferation of A. flexuosa commonly seen as a response to continuous N additions, also occurs already a few months following one single N application, independent of the N form added. The strongest increase in A. flexuosa was, however, seen the year after the N additions when the abundance was three times higher on both high AN and high ARG plots than on control plots. An effect of N dose was apparent as the abundance was significantly higher in high N plots than in low N plots although it was not proportional, i.e. although the N dose was three times higher on high N than on low N plots the A. flexuosa abundance was only c 30% higher on the high N plots. Five years after the N additions the abundance of A. flexuosa had declined, but was still higher on N addition plots than on control plots with a remaining N dose effect only on ARG plots. The very similar responses of A. flexuosa to AN and ARG addition was surprising since previous N uptake studies have shown that while this species has a very high capacity to proliferate on inorganic N and in particularly on nitrate, it does not readily take up ARG (Persson et al. 2003; Nordin et al. 2006). However, following ARG addition high levels of ammonium was retained on ion exchange capsules suggesting that ammonium could have been a major N source for A. flexuosa. A decreased abundance of A. flexuosa over time has been observed also in other N addition experiments (i.e. Nordin et al. 2009) and may be due to both reinstated N limitation and suppression from N enhanced growth of the tree canopy (Strengbom et al. 2001).
In contrast to the immediate response to N addition displayed by A. flexuosa, a negative effect from the N additions on the abundance of the dwarf shrub V. vitis-idaea was detected only after five years. Studies have shown that light and moisture conditions are more important than N supply in explaining the abundance of V. vitis-idaea (Mäkipää et al. 1999; Palmroth et al. 2014) and we suggest that the long-term negative response of this species to the N additions is mainly due to tree canopy suppression. For the bryophyte dominating the site, P. schreberi, an immediate negative effect of N addition was expected as bryophytes have a high capacity to take up N, particularly as ammonium and amino acids (Forsum et al. 2006; Wiedermann et al. 2009), and bryophyte decline in high N environments have been repeatedly reported (Hedwall et al. 2010; Verhoeven et al. 2011; Gundale et al. 2013) and subjected either to direct toxic effects or to increased competition from vascular plants (Turetsky et al. 2012 and references therein). Due to the absent immediate effect on bryophyte abundance, it may be that the observed long-term decline of P. schreberi was more related to suppression from competing vegetation than to any immediate toxic effects from the N additions.
Understanding how N addition affects structures and functions directing northern ecosystems’ long-term capacity for carbon sequestration is key to fully apprehend how management measures, like N additions, can be directed to interlink N and carbon dynamics for effective climate change mitigation (Mack et al. 2004; Janssens et al. 2010; Maaroufi et al. 2015). The less negative effect of ARG compared to AN on EcM fungi is interesting in this perspective as C derived from EcM fungi may remain more recalcitrant than C input via plant litter (Clemmensen et al. 2013, 2015). Our short-term result showing different EcM responses to ARG and AN additions merits further investigations revealing the responsible mechanisms incorporating ARG and AN in different types of forest ecosystems before making conclusions on the long-term large-scale ecosystem effects.
References
Avis PG, McLaughlin DJ, Dentinger BC, Reich PB (2003) Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp in a temperate oak savanna. New Phytol 160:239–253
Avolio ML, Tuininga AR, Lewis JD, Marchese M (2009) Ectomycorrhizal responses to organic and inorganic nitrogen sources when associating with two host species. Mycol Res 113:897–907
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57(1):289–300
Berch SM, Brockley RP, Battigelli J, Hagerman S (2006) Impacts of repeated fertilization on components of the soil biota under a young lodgepole pine stand in the interior of British Columbia. Can J For Res 36:1415–1426
Berch SM, Brockley RP, Battigelli J, Hagerman S (2009) Impacts of repeated fertilization on fine roots, mycorrhizas, mesofauna, and soil chemistry under young interior spruce in central British Columbia. Cad J For Res 39:889–896
Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For Ecol Manag 119:51–62
Binkley D, Burnham H, Allen HL (1999) Water quality impacts of forest fertilization with nitrogen and phosphorus. For Ecol Manag 121:191–213
Brockley RP (2010) Effects of repeated fertilization in a young spruce stand in central British Columbia. Can J Fort Res 40:1687–1697
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in Boreal forest. Science 39:1615–1618
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD (2015) Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol 205:1525–1536
Core Team R (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria URL http://wwwR-projectorg/
Forsum Å, Dahlman L, Näsholm T, Nordin A (2006) Nitrogen utilization by Hylocomium splendens in a boreal forest fertilization experiment. Funct Ecol 20:421–426
Fransson PMA, Taylor AFS, Finlay RD (2000) Effects of continuous optimal fertilization on belowground ectomycorrhizal community structure in a Norway spruce forest. Tree Physiol 20:599–606
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forest. For Ecol Manag 196:159–171
From F, Strengbom J, Nordin A (2015) Residual long-term effects of forest fertilization on tree growth and nitrogen turnover in boreal forest. Forests 6:1145–1156
From F, Lundmark T, Mörling T, Pommerening A, Nordin A (2016) Effects of simulated long-term N deposition on Picea abies and Pinus sylvestris growth in boreal forest. Can J For Res 46:1396–1403
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gruffman L, Ishida T, Nordin A, Näsholm T (2012) Cultivation of Norway spruce and Scots pine on organic nitrogen improves seedling morphology and field performance. For Ecol Manag 276:118–124
Gruffman L, Jämtgård S, Näsholm T (2014) Plant nitrogen status and co-occurrence of organic and inorganic nitrogen sources influence root uptake by Scots pine seedlings. Tree Physiol 34:205–213
Gruffman L, Palmroth S, Näsholm T (2013) Organic nitrogen uptake of Scots pine seedlings is independent of current carbohydrate supply. Tree Physiol 33:590–600
Gundale MJ, Bach LH, Nordin A (2013) The impact of simulated chronic nitrogen deposition on the biomass and N2-fixation activity of two boreal feather moss – cyanobacteria associations. Biol Lett 9:20130797
Gundale MJ, From F, Bach LH, Nordin A (2014) Anthropogenic nitrogen deposition in boreal forests has a minor impact on the global carbon cycle. Glob Chang Biol 20:276–286
Hasselquist NJ, Högberg P (2014) Dosage and duration effects of nitrogen additions on ectomycorrhizal sporocarp production and functioning: an example from two N-limited boreal forests. Ecol Evol 4:3015–3026
Hay TN, Phillips LA, Nicholson BA, Jones MD (2015) Ectomycorrhizal structure and function in interior spruce forest of British Columbia under long term fertilization. For Ecol Manag 350:87–95
Hedwall PO, Nordin A, Brunet J, Bergh J (2010) Compositional changes of forest-floor vegetation in young stands of Norway spruce as an effect of repeated fertilization. For Ecol Manag 259:2418–2425
Hedwall PO, Strengbom J, Nordin A (2013) Can thinning alleviate negative effects of fertilization on boreal forest floor vegetation? For Ecol Manag 310:382–392
Hothorn T, Westfall P (2008) Simultaneous inference in General Parametric Models. Biom J 50:346–363
Inselsbacher E, Näsholm T (2012) The below-ground perspective of forest plants: soil provides mainly organic nitrogen for plants and mycorrhizal fungi. New Phytol 195(2):329–334
Ishida TA, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer–broadleaf forests. New Phytol 174(2):430–440
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jones DL, Kielland K (2002) Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol Biochem 34:209–219
Jones MD, Phillips LA, Treu R, Ward V, Berch SM (2012) Functional responses of ectomycorrhizal fungal communities to long-term fertilization of lodgepole pine (Pinus contorta Dougl. ex Loud. var. latifolia Engelm.) stands in central British Columbia. Appl Soil Ecol 60:29–40
Kielland K, McFarland JW, Ruess RW, Olson K (2007) Rapid cycling of organic nitrogen in taiga forest ecosystems. Ecosystems 10:360–368
Kõljalg U, Larsson KH, Abarenkov K, Nilsson RH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T, Sen R, Taylor AFS, Tedersoo L, Vrålstad T, Ursing BM (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068. https://doi.org/10.1111/j.1469-8137.2005.01376.x
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11:397–410
Maaroufi N, Nordin A, Hasselquist NJ, Bach LH, Palmqvist K, Gundale M (2015) Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Glob Chang Biol 21:3169–3180
Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS III (2004) Ecosystem carbon storage in arctic tundra reduced by lont-term nutrient fertilization. Nature 431:440–443
Mäkipää R, Karjalainen T, Pussinen A, Kellomäki S (1999) Effects of climate change and nitrogen deposition on the carbon sequestration of a forests ecosystem in the boreal zone. Can J For Res 29:1490–1501
Manninen OH, Tolvanen A (2013) N-fertilization and disturbance impacts and their interaction in forest-tundra vegetation. Plant Ecol 214:1505–1516
Marschner H, Häussling M, George E (1991) Ammonium and nitrate uptake rates and rhizosphere pH in non-mycorrhizal roots of Norway spruce Picea abies (L) Karst. Trees 5:14–21
McFarland JW, Ruess RW, Kielland K, Pregitzer K, Hendrick R, Allen M (2010) Cross-Ecosystem Comparisons of In Situ Plant Uptake of Amino Acid-N and NH4 +. Ecosystems 13:177–193
Melin J, Nommik H (1988) Fertilizer nitrogen distribution in a Pinus sylvestris / Picea abies ecosystem central Sweden. Scand J For Res 3:3–15
Nadelhoffer KJ, Downs MR, Fry B (1999) Sinks for 15N-enriched additions to an oak forest and a red pine plantation. Ecol Appl 9(1):72–86
Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916
Nohrstedt HÖ (2001) Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scand J For Res 16:555–573
Nordin A, Högberg P, Näsholm T (2001) Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 129:125–132
Nordin A, Näsholm T, Ericson L (1998) Effects of simulated N deposition on understorey vegetation of a boreal coniferous forest. Funct Ecol 12:691–699
Nordin A, Strengbom J, Ericson L (2006) Responses to ammonium and nitrate additions by boreal plants and their natural enemies. Environ Pollut 141:167–174
Nordin A, Strengbom J, Forsum Å, Ericson L (2009) Complex biotic interactions drive long-term vegetation change in a nitrogen enriched boreal forest. Ecosystems 12:1204–1211
Öhlund J, Näsholm T (2001) Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol 21:1319–1326
Öhlund J, Näsholm T (2002) Low nitrogen losses with a new source of nitrogen for cultivation of conifer seedlings. Environ Sci Technol 36:4854–4859
Oyewole OA, Jämtgård S, Gruffman L, Inselsbacher E, Näsholm T (2016) Soil diffusive fluxes constitute the bottleneck to tree nitrogen nutrition in a Scots pine forest. Plant Soil 399:109–120
Palmroth S, Holm-Bach L, Nordin A, Palmqvist K (2014) Nitrogen-addition effects on leaf traits and photosynthetic carbon gain of boreal forest understory shrubs. Oecologia 175:457–470
Persson J, Högberg P, Ekblad A, Högberg MN, Nordgren A, Näsholm T (2003) Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137:252–257
Peter M, Ayer F, Egli S (2001) Nitrogen addition on a Norway spruce stand altered macromycete sporocarp production and below-ground ectomyccorhizal species composition. New Phytol 149:311–325
Saarsalmi A, Mälkönen E (2001) Forest fertilization research in Finland: a literature review. Scand J For Res 16:514–535
Scott EE, Rothstein DE (2011) Amino acid uptake by temperate tree species characteristic of low- and high-fertility habitats. Oecologia 167:547–557
Strengbom J, Nordin A (2008) Commercial forest fertilisation causes long-term residual effects in ground vegetation of boreal forests. For Ecol Manag 256:2175–2781
Strengbom J, Nordin A (2012) Physical disturbance determines effects from nitrogen addition on ground vegetation in boreal coniferous forests. J Veg Sci 23:361–371
Strengbom J, Nordin A, Näsholm T, Ericson L (2001) Slow recovery of boreal forest ecosystem following decreased nitrogen input. Funct Ecol 15:451–457
Suz LM, Barsoum N, Benham S, Cheffings C, Cox F, Hackett L, Jones AG, Mueller GM, Orme D, Seidling W, Van der Linde S, Bidartondo MI (2015) Monitoring ectomycorrhizal fungi at large scale for science forest management fungal conservation and environmental policy. Ann For Sci 72:877–885
Templer PH, Mack MC, Chapin IIIFS, Christenson LM, Compton JE, Crook HD, Currie WS, Curtis CJ, Dail DB, CM D’A, Emmett BA, Epstein HE, Goodale CL, Gundersen P, Hobbie SE, Holland K, Hooper DU, Hungate BA, Lamontagne S, Nadelhoffer KJ, Osenberg CW, Perakis SS, Schleppi P, Schimel J, Schmidt IK, Sommerkorn M, Spoelstra J, Tietema A, Wessel WW, Zak DR (2012) Sinks for nitrogen inputs in terrestrial ecosystems: a meta-analysis of 15N tracer field studies. Ecology 93:1816–1829
Turetsky MR, Bond-Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD, Tuittila E-S (2012) The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol 196:49–67
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York 0-387-95457-0
Verhoeven JTA, Beltman B, Dorland E, Robat SA, Bobbink R (2011) Differential effects of ammonium and nitrate deposition on fen phanerogams and bryophytes. Appl Veg Sci 14:149–157
Wallander H, Arnebrant K, Östrand F, Kårén O (1997) Uptake of 15N-labelled alanine ammonium and nitrate in Pinus sylvestris L ectomycorrhiza growing in forest soil treated with nitrogen sulphur or lime. Plant Soil 195:329–338
White TJ, Bruns T, Lee SJWT, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a Guide to Methods and Applications 18:315–322
Wiedermann MM, Gunnarsson U, Ericsson L, Nordin A (2009) Ecophysiological adjustment of two Sphagnum species in response to antropogenic nitrogen deposition. New Phytol 181:208–217
Acknowledgements
This research was supported by the Future Forests, an interdisciplinary research program funded by MISTRA, the Swedish University of Agricultural Sciences, Umeå University, the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (AN and TN), and JSPS Postdoctoral Fellowships for Research Abroad (TAI) We are grateful to Ann Sehlstedt, Sonja Vahlberg and Lisa Edvardsson for field and laboratory works
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap
Electronic supplementary material
ESM 1
(DOCX 71 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hedwall, PO., Gruffman, L., Ishida, T. et al. Interplay between N-form and N-dose influences ecosystem effects of N addition to boreal forest. Plant Soil 423, 385–395 (2018). https://doi.org/10.1007/s11104-017-3444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3444-1