Abstract
Aims
Ectomycorrhizal fungi can improve poplar growth and tolerance to heavy metal stress, and may be useful during the afforestation and phytoremediation of polluted regions with poplar trees. In this study, we determined the effects of the symbiotic interaction between Populus × canescens trees and Paxillus involutus strains different in their tolerance to lead.
Methods
In vitro inoculated and non-inoculated plants were treated with 0.75 mM Pb(NO3)2. The root colonization rate of the two fungal strains, as well as their impacts on poplar health and lead accumulation were examined.
Results
Based on the colonization level, the roots were classified into one of three categories: non-mycorrhized, changed (ie, fungal cells were present on the root surface, but the Hartig net did not fully develop), and fully mycorrhized. The lead-tolerant P. involutus strain colonized roots better than the non-tolerant strain (ie, changed and fully mycorrhized roots). Moreover, plants inoculated with the tolerant fungal strain grew better than the control plants (217 % increase in dry weight over the controls), and accumulated lead in the roots and stems.
Conclusions
Inoculation of P. × canescens trees with a Pb-tolerant strain of P. involutus improves host plant growth and may increase Pb phytostabilization potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ectomycorrhizal (ECM) fungi are obligatory symbionts of vascular plants, including poplar trees (Smith and Read 2008). These fungi belong to various taxonomic groups, with the majority in the phyla Ascomycota and Basidiomycota (Krpata et al. 2008). Paxillus involutus is an example of an ECM fungus from the phylum Basidiomycota (Smith and Read 2008). Unlike arbuscular mycorrhizal symbionts, ECM fungi do not penetrate host cells. Instead, they form a mantle that surrounds the roots, and penetrate between the epidermis and cortical cells to produce fully functional ectomycorrhizae (ie, the Hartig net) (Smith and Read 2008). It is here that compounds are exchanged between the plant host and fungi. Plants receive nutrients, especially nitrogen (Willmann et al. 2014) and phosphorus (Vodnik et al. 1996), as well as water (Marjanović et al. 2005) in exchange for carbohydrates. Consequently, nutrient contents increase in plant host tissues (Vodnik et al. 1996; Ma et al. 2014). However, approximately 20 % of the carbon assimilated by the plant is consumed by the fungal symbiont for its external mycelia (Cairney 2012). Nevertheless, in most cases, ECM fungi significantly increase the plant host biomass (Danielsen et al. 2013; Ma et al. 2014). This has economic implications for poplar trees, which serve as valuable sources of biomass (Szuba 2015). Mycorrhizae are important, especially in nutrient-deficient regions (Krpata et al. 2008) and anthropogenically disturbed environments (Krpata et al. 2008; Karliński et al. 2013; Willmann et al. 2014). Moreover, Populus spp., particularly aspens, are early-successional trees (Krpata et al. 2008; Szuba 2015). Because of their potential ability to grow in harsh conditions, poplar trees with mycorrhizal associations may be useful for the phytoremediation of regions polluted with heavy metals, particularly lead (Bhargava et al. 2012; Ali et al. 2013).
Lead is one of the biggest threats to the environment. Because lead can precipitate, once an area is affected, it remains polluted (Tangahu et al. 2011; Fahr et al. 2013). Therefore, various methods for soil remediation, including ECM fungi-mediated phytoremediation, are highly sought after (Ali et al. 2013). Lead belongs to a group of non-essential heavy metals, which are toxic to living organisms even at very low concentrations because they accumulate in tissues. In excessive amounts, lead causes abnormal plant cell division, altered nitrogen metabolism, disorders in plant-water relationships, and inhibited growth and enzymatic activities (Fahr et al. 2013). Heavy metals damage cellular membranes, proteins, lipids, and DNA (Michalak 2006; Jiang and Liu 2010). However, plants respond to lead exposure by adsorbing Pb2+ ions in the cell wall, predominantly by low-methyl esterified pectins (Rabęda et al. 2015) and other cell wall compounds such as hemicellulose and phenols (Krzesłowska 2011), transporting Pb2+ ions to protoplasts (mainly vacuoles) using thiol-containing groups, and activating antioxidant systems (Bellion et al. 2006). Most Pb2+ ions are bound to cation exchange sites and immobilized in roots (Jentschke and Godbold 2000). Therefore, damages are caused by high cytosolic concentrations of lead (Jiang and Liu 2010).
The symbiotic relationship between plants and mycorrhizal fungi results in enhanced host tolerance to various abiotic stresses, including exposure to heavy metals (Jentschke and Godbold 2000; Szuba 2015). This tolerance is associated with the immobilization of lead compounds in the rhizosphere with organic compounds, such as peptides or organic acids, secreted by the fungi (Turnau et al. 2006; Johansson et al. 2008). Consequently, a lower abundance of lead is available for plant roots. Fungal detoxification and storage involve the binding of bioavailable Pb2+ ions to fungal cell wall elements, such as chitin (Marschner et al. 1998; Jentschke and Godbold 2000), or their chelation and transport to vacuoles (Bellion et al. 2006). Hence, fungal mycelia form a barrier that protects plant tissues from the toxic effects of Pb2+ ions (Marschner et al. 1998; Bellion et al. 2006). However, the mechanisms regulating ECM fungi-mediated responses to heavy metal stress have not been fully characterized.
In general, heavy metals inhibit the growth of ECM fungi (Vodnik et al. 1998; Blaudez et al. 2000). However, the lead toxicity threshold in ECM fungi is one order of magnitude higher than that of their plant hosts. This may explain why plants with mycorrhizal associations exhibit increased tolerance to heavy metals (Vodnik et al. 1998). Additionally, the presence of ECM fungi limits the decrease in host plant biomass caused by heavy metals (Schützendübel and Polle 2002; Ma et al. 2014), with the extent of the protection dependent on the symbiotic partners (Szuba 2015). Thus, a thorough evaluation of the effects of fungal species on host poplar trees is required to ensure optimal fungal strains are used for the phytostabilization of soils or for improving poplar growth in unpolluted plantations (Maltz and Treseder 2015). The aim of this study was to determine the effects of the inoculation of Populus × canescens trees with two strains of P. involutus that differ in their tolerance to lead. The influence of these fungal strains on the effects of Pb2+ on poplar trees was investigated under controlled, in vitro conditions to avoid cross-contaminations with other fungal strains, which is a possibility under natural conditions (Szuba 2015).
Materials and methods
Poplar trees and fungal cultures
Populus × canescens in vitro cultures were kindly provided by Prof. K. Bojarczuk from the Institute of Dendrology, Polish Academy of Sciences, Poland. Poplar trees were cultivated in glass jars containing full-strength Murashige and Skoog medium (Murashige and Skoog 1962) supplemented with myo-inositol (100 mg l−1), 1-naphthaleneacetic acid (0.05 mg l−1), and 6-benzylaminopurine (0.1 mg l−1). The medium also contained 1.5 % sucrose and 0.8 % agar. The pH was adjusted to 5.5 before the medium was autoclaved. Poplar cultures were incubated in a growth chamber at 21 °C and 60 % relative humidity with a 16-h/8-h day/night photoperiod using cool white fluorescent light (150 μmol m−2 s−1).
Fungal strains were cultured on Modified Melin-Norkrans medium (Kottke et al. 1987). They were isolated from fruiting bodies harvested under poplar trees (refer to Online Resource 1 for details regarding in vitro generation and selection of fungal strains). The two P. involutus strains selected for subsequent inoculations of P. × canescens trees were the least and most tolerant to 75 mM Pb(NO3)2. The strain with the lowest tolerance (ie, non-tolerant strain) originated from uncontaminated areas, and had a tolerance index of 42. The most tolerant strain originated from areas surrounding a copper smelter (Głogów, Poland), and had a tolerance index of 109 (for details on the calculation of the tolerance indices, see Online Resource 1).
Experimental design
In April 2014, P. × canescens microcuttings with 2-cm-long roots were prepared. The roots were shortened to 0.5 cm and transferred to new jars containing a 3-mm layer of Modified Melin-Norkrans medium on top of Murashige and Skoog medium. For inoculations, mycelial fragments (5-mm diameter) from one of the two fungal strains were placed near the freshly transferred poplar microcuttings. Inoculated and non-inoculated samples (Online Resource 1) were grown for 6 weeks on the control medium or medium supplemented with 0.75 mM Pb(NO3)2. Control plants were treated with 0.75 mM NH4NO3. Each treatment was completed using 18 plants.
Mineral analysis including lead accumulation
All leaves, stems and roots were dried separately at 60 C° for 48 h to obtain their dry weights. Pooled samples of dried leaf (100 mg) stem (40 mg) and root (10 mg; for lead analysis only) were ground in a ball mill to a powder, mineralized and analyzed for Na, P, K, Mg, Ca, Fe, Cr, Sr, V, Mn, Cu, Zn, and Pb concentrations by using a microwave mineralizer (Multiwave 3000, Anton Paar, GmbH, Austria) and inductively coupled plasma time-of-flight (TOF) mass spectrometer (Spectrometer OptiMass 9500 ICP-TOF-MS, GBC Scientific Equipment, Hampshire, Braeside, Australia). The following certified reference materials were used for all runs: NCS DC 73349 Bush Branches and Leaves [China National Analysis Center for Iron & Steel, Beijing, China; certificate value for Pb: CV(Pb) =47 ± 3; for the other CV values see Online Resource 6] or NIST 1515 Apple Leaves (National Institute of Standards and Technology, Gaithersburg, Md; CV(Pb) =0.470 ± 0.024). All elemental analyses were performed in six replicates at the Institute of Dendrology, Polish Academy of Sciences, Kórnik, Poland. Additionally, the solubilities of Pb compounds in aqueous solution of pure Murashige and Skoog culture medium were calculated with Medusa software (Puigdomenech, Medusa software, KTH University Sweden).
Root colonization analysis
The extent of root colonization was determined for inoculated plants grown in control or lead-supplemented medium (n = 10) using an Axioscope 20 microscope (Carl Zeiss, Jena, Germany). Root tips were counted and classified into one of three categories: non-mycorrhized, changed (by the presence of fungi, but lacking a fully mycorrhized tip), and fully mycorrhized.
Representative root tips for each colonization category were fixed in 4 % paraformaldehyde (Polysciences, Warrington, PA, USA) and 4 % glutaraldehyde (Polysciences) in 0.1 M sodium cacodylate buffer for 24 h at room temperature. Samples were then washed in 0.05 M sodium cacodylate buffer. The root tips were dehydrated in an ascending series of ethanol concentrations, and then embedded in Technovit 7100 resin (Heraeus Kulzer, Wehrheim, Germany). An RM2265 microtome (Leica, Wetzlar, Germany) was used to obtain 40-μm cross sections, which were cut 2 mm from the root tip. To visualize the penetration of hyphae into the roots, cross sections were stained with 0.05 % toluidine blue O (Sigma, St Louis, MO, USA) in 1 % sodium tetraborate buffer, and then analyzed microscopically. Additionally, the diameters of the mycelial growth in jars (n = 18) and in pure cultures (n = 6) were measured using ImageJ v1.48 software (Wayne Rasband, Bethesda, MD, USA).
Biometric analysis of roots
Roots of 18 plants per treatment were carefully cleaned to remove agar medium. Roots were scanned with a high resolution Epson Perfection V700 photo scanner, and analyzed using WinRhizo software (Régent Instruments Inc. Quebec, Canada). The whole root length, projected area (PA), and average diameter were measured. Root dry weight (DW) was used to determine DW% [DW/FW (fresh weight) × 100], and specific root length (root length per root biomass; SRL).
Biometric analysis of aerial plant tissues
Leaves from five randomly selected plants per treatment were scanned, and the resulting images were processed with WinFolia software (Régent Instruments Inc. Quebec, Canada). The number of leaves per plant, and leaf area, perimeter, vertical length, horizontal width (highest and average values), and width:length ratio were calculated. Leaf DW was used to determine DW% and specific leaf area (leaf area per leaf biomass; SLA). Additionally, the length, DW, and DW% of stems were determined (n = 18). The DW of leaves, stems, and roots were used to calculate whole-plant biomass and the shoot:root ratio.
Chlorophyll measurements
Chlorophyll concentrations were calculated for pooled leaf samples (n = 6) using a photospectrometry-based acetone method (Lichtenthaler and Wellburn 1983) as described by Szuba and Lorenc-Plucińska, (2015). Chlorophyll a (Chl a), chlorophyll b (Chl b) and chlorophyll (x + c) [Chl (x + c); total carotenoids] concentrations were determined (mg g−1) for fresh and dried leaves.
Statistical analyses
All results are presented as the mean ± standard error. Data were analyzed using STATISTICA 5 (StatSoft Inc., Tulsa, OK, USA). Analysis of variance was used to compare all samples. Means were compared using the Tukey’s honest significance test. Differences were considered significant at P < 0.05. The tolerance indices were calculated according to Lorenc-Plucińska et al. (2013), for details, see Online Resource 1.
Results
Inoculation with either P. involutus stain significantly affected the plant host under control conditions as well as under Pb treatment conditions. Representative images of analyzed variants are presented in Fig. 1.
Representative images of analyzed plants: (a) and (d) non-inoculated plants; (b) and (e) plants inoculated with the non-tolerant fungal strain; (c) and (f) plants inoculated with the Pb-tolerant fungal strain. Images a–c correspond to control conditions, while images d–f correspond to lead-treatment conditions (0.75 mM Pb(NO3)2)
Root colonization analysis
Morphological and anatomical characteristics of inoculated roots
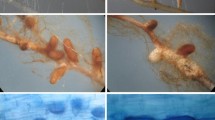
Both P. involutus strains grew on agar medium (for details, see Online Resources 2 and 3) and infected roots. After 6 weeks of cultivation with fungal strains, the root tips were divided into three categories (Figs. 2 and 3). This division was based on the morphological features of the root tips (Fig. 2), and was confirmed by their anatomical structures (Fig. 3; example of root tips inoculated with the tolerant fungal strain growing under control conditions). In non-mycorrhized root tips, numerous root hairs were visible (Fig. 2a). The non-inoculated roots exhibited typical structures for the epidermis, cortical cells and stele (Fig. 3a). The changed roots became brownish (Fig. 2b–d), and in some cases were thicker and shorter than the non-mycorrhized roots (Fig. 2b). Root hairs were observed in some plants (Fig. 2c), and most roots were long (Fig. 2c and d). However, considerable changes were observed mainly in the root tips (Fig. 2d). Anatomical analysis revealed that the fungal cells in changed roots were usually present on the root surface, where they formed mantles (Fig. 3c and d). Moreover, single fungal cells were detected between plant cortical cells (Fig. 3c and d), which likely indicated the initial stages of the formation of the Hartig net. Finally, fully mycorrhized root tips were thicker and shorter than the other root tips, and lacked root hairs (Fig. 2e; Szuba, 2015), but contained well-developed fungal mantles (Fig. 3d) and Hartig nets (Fig. 3d).
Anatomical structures of root tips. Microscopy images of cross sections of fixed poplar root tips from control plants inoculated with the lead-tolerant Paxillus involutus strain. (a) Non-mycorrhized roots. (b) Changed root tips with a mantle, but no fungal cells, between cortical cells. (c) Changed root tips with a mantle and individual fungal cells present in the cortex. (d) Fully mycorrhized root tips. E, epidermis; C, cortical cells; S, stele; asterisk, fungal mantle; arrowheads, Hartig net. Scale bar = 100 μm
Root colonization by fungal strains
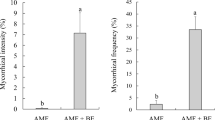
Based on the percentage of fully mycorrhized and changed root tips colonized by fungi (Fig. 4a), the colonization rate of the tolerant strain was significantly higher than that of the non-tolerant strain, both in control conditions and in the presence of Pb2+ ions. The percentage of roots colonized by the tolerant strain was more than 5-fold higher than that of roots colonized by the non-tolerant strain (Fig. 4a).
Among the inoculated samples, the average number of root tips (ie, sum of non-mycorrhized, changed, and fully mycorrhized root tips) was higher for the tolerant fungal strain. However, significant differences (P = 1.3e-04) were observed only for plants exposed to lead (Fig. 4b).
Biometric data
The DW of whole plants inoculated with the tolerant fungal strain was significantly higher than that of the non-inoculated plants (P = 8.6e-05; Fig. 5a). Under control conditions, the DW of inoculated plants was 155 % higher than that of non-inoculated plants. In plants exposed to lead, the DW of inoculated plants was 213 % higher than that of non-inoculated plants. A similar trend was observed for plants inoculated with the non-tolerant strain, but the differences were not significant (P = 0.26; Fig. 5a). In non-inoculated samples, the DW of control plants was significantly higher than that of plants exposed to lead (P = 0.029). Conversely, in plants inoculated with the tolerant P. involutus strain, the DW of lead-exposed materials was similar to that of the controls (Fig. 5a). In plants inoculated with the non-tolerant fungal strain, the DW of lead-treated tissues was lower than that of the controls, but not significantly (P = 0.061). The differences between the lead-treated plants and controls were not as large as the differences between non-inoculated plants.
There were no differences in the shoot:root ratios among plants grown in the control medium (Fig. 5b). In contrast, in the presence of Pb2+ ions, the shoot:root ratios were significantly higher in non-inoculated plants than in plants inoculated with the tolerant fungal strain (P = 0.019; Fig. 4b).
Roots
Under control and lead treatment conditions, root DW was significantly higher (P = 4.3e-04) for plants inoculated with the tolerant fungal strain than for the non-inoculated plants (Fig. 5d). Total root length did not differ significantly among all plants (P = 0.11); however the average root diameters were larger in inoculated plants than in the non-inoculated samples, especially in plants inoculated with the tolerant fungal strain (details regarding changes in other biometric parameters of roots are presented in Online Resource 4). There were no significant differences in root DW between plants grown under control or lead treatment conditions. However the average values for root DW tended to be higher following lead exposure for plants inoculated with the tolerant fungal strain, while the opposite trend was observed for plants inoculated with the non-tolerant strain (Fig. 5d). Similar trends were observed for the other root parameters (for details on length, PA, diameter, and DW, see Online Resource 4a). The roots most influenced by mycorrhizal associations were root categories with a diameter of 0.3–0.6 mm or 0.6–0.9 mm, where both fungal strains induced similar increases in length and PA (for details, see Online Resource 4b).
In general, the tolerant fungal strain affected root architecture (especially in plants exposed to lead) more than the non-tolerant strain. Both fungal strains considerably affected root architecture, while lead ions did not.
Stems
Inoculated poplar trees were taller than the non-inoculated ones (Fig. 1 and Fig. 5c). Additionally, stem mass increased significantly (P = 3.9e-04) only in plants treated with the tolerant fungal strain (Fig. 5e). There were no significant differences in height and stem mass between plants grown under control or lead treatment conditions.
Leaves
Under control conditions, leaf mass from plants inoculated with the Pb-tolerant P. involutus strain was significantly higher (P = 0.001) than that of non-inoculated plants. The mass of leaves from plant inoculated with the non-tolerant strain was intermediate between those of the other two groups. Lead treatment caused a non-significant decrease in leaf mass (Fig. 5f). However expanded morphometric analysis suggested that the shapes of leaves from stressed plants were similar to those of controls, but the leaves were smaller (see Online Resource 5 for details). Otherwise, fungal inoculation did not significantly affect the leaves’ biometric parameters in control or stressed plants. However, a tendency for increased leaf size was observed with inoculation (see Online Resource 5).
Chlorophyll measurements
The Chl a concentrations of dried plant tissue were not influenced by either fungal strain under control conditions (Fig. 6a). However, in fresh leaves harvested from plants inoculated with the tolerant fungal strain, a significant increase in Chl a abundance was observed (P = 1.4e-04; Fig. 6d). The concentration of Chl b in fresh leaves increased considerably for both fungal strains (Fig. 6b). Similar to the Chl a and b concentrations, the carotenoid concentrations depended on how they were measured. When calculated using dried plant material, the Chl (x + c) concentration decreased in the leaves of plants inoculated with the tolerant fungal strain (under control conditions). In contrast, when fresh leaf material was used, carotenoid levels increased significantly (P = 1.4e-04) relative to the levels of non-inoculated plants.
Lead generally did not decrease leaf pigment content (when comparing plants under control or lead treatment conditions) in dried and fresh leaves. In fact, Chl a and Chl (x + c) concentrations from dried leaves were significantly higher (P = 0.042 and P = 0.016, respectively) in non-inoculated plants grown in the presence of lead ions (Fig. 6a and c). Similar tendencies were observed for plants inoculated with the non-tolerant fungal strain. In contrast, plants inoculated with the tolerant strain experienced a non-significant decrease in the average Chl a and Chl (x + c) concentrations under control and lead treatment conditions. These opposing trends in the response to lead ions (in dried leaves) resulted in bigger differences between inoculated plants grown in the presence of heavy metals. The concentrations of Chl a, Chl b and carotenoids were lowest in plants (exposed to lead) inoculated with the tolerant fungal strain (Fig. 6a–c).
Mineral composition and lead accumulation
Inoculation with both P. involutus strains caused considerable changes to mineral content in aerial tissues of plants grown under control conditions. This was particularly noticeable for plants inoculated with the tolerant fungal strain (Online Resource 6), in which the concentration of almost all elements decreased significantly. This was in contrast with the results of plants inoculated with the non-tolerant strain, in which the concentrations of some elements (eg, P and Mg) were higher than in non-inoculated plants. The abundance of most analyzed elements in the leaves and stems of plants exposed to lead remained stable or increased (Online Resource 6).
According to calculations using Medusa software, in aqueous solution of pure Murashige and Skoog medium, Pb creates 15 solid complexes with high proportion of Pb5(PO4)3Cl. However, in the presence of plants, Pb was partially bio-available because it was absorbed by roots. The lead concentrations (μg g−1) in dried roots were highest in non-inoculated plants, and lowest in plants inoculated with the non-tolerant fungal strain (Fig. 7a). Additionally, the highest lead contents (Fig. 7b) were observed in plants inoculated with the tolerant P. involutus strain. The lead concentration and content in stems (Fig. 7) were highest in plants inoculated with the tolerant fungal strain, followed by plants inoculated with the non-tolerant strain. The leaf lead concentration (Fig. 7) was lowest in plants inoculated with the tolerant P. involutus strain.
Dry matter concentrations
Several of the results described above and in Online resource 4a were likely affected by the dry matter contents. The DW% (Online Resource 7) was highest in plants inoculated with the tolerant fungal strain under control and lead treatment conditions. The increase was highest in roots (175 %), followed by stems (160 %) and leaves (157 %). Low hydration levels of inoculated roots were partly caused by lower hydration levels of fungal tissues present in the mantle. The DW% value was about 15 % for both fungal strains (ie, pure fungal cultures; data not shown), which was 3-fold higher than that of non-inoculated roots.
Discussion
Influence of fungi on poplar microcuttings grown under control conditions
Samples were analyzed 6 weeks after inoculation, by which time the Hartig net is usually present (Tschaplinski et al. 2014). This has also been confirmed for P. × canescens inoculated with P. involutus (Gafur et al. 2004). Our results revealed that the poplar root tips were in various stages of the symbiotic interaction with the fungus (Felten et al. 2012) and mycorrhization was incomplete (Tschaplinski et al. 2014). Up to 100 % of poplar roots may become colonized (Danielsen et al. 2013; Szuba, unpublished data).
The hyphal network penetrates the root cortex, which is the primary site where substances are exchanged between symbiotic partners (Felten et al. 2012; Szuba 2015). Simultaneously, the mantle is formed, and makes the host dependent on the fungal partner because it prevents root hairs from forming and inhibits root growth (Felten et al. 2012; Szuba 2015). It is possible that an under-developed Hartig net and lack of root hairs in changed roots impair nutrient flow in the host. In fact, we observed a decrease in the abundance of almost all analyzed elements in the leaves and stems of plants inoculated with the tolerant fungal strain, in which about 40 % of the root tips were classified as changed. However, most of the biometric parameters of these plants were significantly improved. This may indicate that increased plant growth adversely affects the symbiotic relationship between plant and fungus (Corrêa et al. 2006), especially before the late stages of colonization (Felten et al. 2012).
The mantle considerably affects root structure (Smith and Read 2008; Szuba 2015). However, the roots from inoculated plants differed significantly from those of the non-inoculated controls, suggesting that root length and diameter were positively influenced by the fungi, but not necessarily exclusively by the root colonization rate. Similar results were obtained for Scots pine (Pinus sylvestris), where ECM fungi improved host growth even without fully developed functional mycorrhizae (Sarjala et al. 2010). The occurrence of host structural changes prior to physical contact between symbiotic partners has been reported (Felten et al. 2009). Various signaling molecules, which contribute to improved host growth such as polyamines (Cicatelli al. 2010; Sarjala et al. 2010; Felten et al. 2012) or phytohormones (particularly auxins; Rudawska and Kieliszewska-Rokicka 1997; Krause et al. 2015) may be responsible for this phenomenon.
One of the most frequently reported effects of ECM fungi is increased photosynthetic activity, which is often manifested by an increase in chlorophyll content (Turgeman et al. 2011; Ma et al. 2014). We observed that leaf pigments were strongly influenced by ECM fungi, but the calculated chlorophyll concentrations were strongly influenced by the dry matter content in leaves, which was reflected by the variability in the results of different methods used. Unfortunately, there may be valid explanations for all potential results. Increases in chlorophyll and carotenoid contents have frequently been reported as an effect of symbiotic relationships (Turgeman et al. 2011). This is consistent with the results of our proteome-level analyses, which indicated the interactions between host plants and ECM fungi increase photosystem activities (as well as stress responses; Szuba, unpublished data). However, unchanged or decreased leaf pigment levels have also been reported for symbiotic interactions between plants and ECM fungi (Mrnka et al. 2012). These effects are correlated with a decrease in the concentration of Mg, which is the main cofactor of chlorophylls. These results are also consistent with the observed adverse effects in plants during the early stages of a symbiotic relationship with ECM fungi, which are complemented by increases in DW% (usually considered an indicator of exposure to stress; Rodríguez et al. 2015) following inoculation with the tolerant fungal strain.
Minimal effects of lead on poplar growth
Lead exposure usually causes decreased plant growth (especially the roots) (Fahr et al. 2013), leaf chlorosis or decreased root colonization rates by mycorrhizal fungi (Chen et al. 2005). Some of these decreased biometric parameters have been observed, mainly in non-inoculated plants. However, we observed that lead had relatively little impact on the growth of non-inoculated poplar trees or trees inoculated with non-tolerant or tolerant P. involutus strains. This suggests poplar trees can tolerate the presence of lead, although they are not considered to be lead hyperaccumulators (Rascio and Navari-Izzob 2011; Tangahu et al. 2011; Bhargava et al. 2012). However, a lack of significant effects on growth may be the result of several factors. One possibility is low bioavailability of Pb2+ ions (Tangahu et al. 2011). The bioavailability of lead is strongly dependent on pH, concentrations of other ions present in the medium [mainly phosphates, chlorides and sulfates that have high affinities for Pb2+ ions (Kopittke et al. 2008)] and organic matter content. However, lead bioavailability is also influenced by the growth medium itself (Magrisso et al. 2009). Even in quartz sand, lead bioavailability is only about 50 %, and it decreases to undetectable levels in soils rich in ferrum (Magrisso et al. 2009). Consequently, similar lead concentrations may affect plants differently depending on the growth medium. The bioavailability of lead in artificial agar media has not been determined, but it is likely that lead ions are partly insoluble in artificial media, as they are under natural conditions, because they precipitate in the presence of phosphate ions (Chen et al. 2009). However, Pb was absorbed by plants and was, thus, at least partially bio-available; this bio-availability probably resulted from the presence of acidic plant exudates, which are known to moderate Pb solubility (Fahr et al. 2013).
Low lead bioavailability may induce hormetic mechanisms (Wang et al. 2010), and may explain why leaf chlorophyll or carotenoid contents increase or remain unaffected. It may also be responsible for the increases in root biometric parameters observed in this study.
Benefits of ectomycorrhizal fungi on plants grown in the presence of lead ions
Lead is a weakly mobile metal, and up to 90 % of the lead absorbed by plants is deposited in the roots (Jentschke and Godbold 2000; Fahr et al. 2013). Plants have developed various defense responses to heavy metal toxicity, including the development of mycorrhizae (Jentschke and Godbold 2000; Szuba 2015). In this study, a lead-tolerant P. involutus strain was isolated from fruiting body harvested from soil contaminated with heavy metals. According to several reports, this strain from a reference ecosystem should be useful for improving plant growth in areas contaminated with lead (Adriaensen et al. 2006; Maltz and Treseder, 2015). This is because it is more tolerant to lead in pure cultures and it promotes the growth of lead-stressed poplar trees better than the non-tolerant strain.
Ectomycorrhizal fungi have multiple effects on the amelioration of lead toxicity. First, lead is immobilized in fungal cells (Marschner et al. 1998; Jentschke and Godbold 2000). Additionally, the number of mycorrhized root tips (level of root colonization) may be responsible for the differences in lead contents between the two fungal strains. Second, ECM fungi may secrete various active compounds capable of binding to Pb2+ ions, or stimulate the production and secretion of host compounds that neutralize Pb2+ ions (Jentschke and Godbold 2000).
The greater abundance of pigmented exudates in the growth medium following exposure to lead (Fig. 1 and Online Resource 2) may be partially explained by the activity of laccases, which catalyze the synthesis of melanins from phenolic substrates (Jacob et al. 2004). Both phenolic (Michalak 2006) and laccase (Jacob et al. 2004) synthesis were reported to be upregulated under heavy metal stress and affected by ectomycorrhization (Schützendübel and Polle 2002, Jacob et al. 2004; Courty et al. 2011). The lower concentration (μg mg−1 DW) of lead in inoculated roots, especially those inoculated with the non-tolerant strain, may have resulted from ECM increasing the production and extraction of phenolic compounds (Schützendübel and Polle 2002; Jacob et al. 2004) or metallothioneins (Courty et al. 2011), which both form complexes with heavy metals. However, effect of mycorrhizae on phenolic compounds has not been fully characterized, and differs among various symbiotic interactions (Sutela et al. 2009). Moreover, some phenolic compounds exhibit anti-microbial activities (Münzenberger et al. 1995), and may delay the mycorrhization process, possibly observed for non-tolerant strain. Finally, mycorrhiza, especially with the Pb-tolerant P. involutus strain, improved the growth of poplar trees exposed to heavy metal stress (Szuba, 2015); this improvement may have been associated with upregulated synthesis of plant (Jouve et al. 2004) or fungal (Zarb and Walters 1996) polyamines.
Poplar trees inoculated with the Pb-tolerant P. involutus strain were characterized in our study by increased root biomass, which accumulated high levels of lead. Paxillus involutus exhibits lower absorptive properties than other ECM fungi (Marshner et al. 1998), likely because it releases large amounts of secondary metabolites, including heavy metal chelators, into the growth medium. These metabolites may bind to metal ions in the soil and stabilize Pb in the rhizosphere (Blaudez et al. 2000). However the ability of P. involutus mycelia to absorb lead ions is still 10-fold higher than that of plants (Vodnik et al. 1998).
It is worth noting that poplar trees inoculated with the tolerant fungal strain accumulated higher amounts of Pb2+ ions than the non-inoculated plants, not only within the roots, but also in the stems. The accumulation of heavy metals in host shoots has been observed in other plants inoculated with ECM fungi (Ma et al. 2014; Bojarczuk et al. 2015). This increased translocation of heavy metals may be positively correlated with the level of root colonization by the ECM fungi, which confirms a role for the fungus (number of mycorrhized root tips) during heavy metal absorption (Jentschke and Godbold 2000).
The lead-tolerant P. involutus strain may also protect photosynthetically active organs from the effects of lead toxicity. This protective activity was observed in the leaves of Robinia pseudoacacia plants grown in the presence of arbuscular mycorrhizal species (Yang et al. 2015). In our study, poplar trees inoculated with the lead-tolerant P. involutus strain had relatively high lead concentrations in the stem, while the leaves contained the lowest lead levels. This may explain why the leaf chlorophyll and carotenoid contents increased or remained unaffected.
Overall, our findings revealed a notable, positive impact of inoculating poplar trees with appropriate ECM fungi on host plant growth and Pb phytostabilization, which is recognized as one of the major types of phytoremediation (Zhao and McGrath 2009; Ijaz et al. 2016). In conclusion, poplar trees inoculated with the lead-tolerant P. involutus strain may exhibit higher phytostabilization potential in terms of tolerance to Pb stress and heavy metal accumulation. Such non-genetic modifications leading to enhanced phytoremediation (Zhao and McGrath 2009; Ijaz et al. 2016) may be useful for the development of effective soil remediation technologies. However, we must consider that the results obtained under controlled environmental conditions may differ from those obtained under field conditions.
Abbreviations
- Chl (x + c):
-
Chlorophyll (x + c) total carotenoids
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- DW:
-
Dry weight
- ECM:
-
Ectomycorrhizal
- FW:
-
Fresh weight
- PA:
-
Projected area
- SLA:
-
Specific leaf area
- SRL:
-
Specific root length
References
Adriaensen K, Vangronsveld J, Colpaert J (2006) Zinc-tolerant Suillus bovinus improves growth of Zn-exposed Pinus sylvestris seedlings. Mycorrhiza 16:553–558
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Bellion M, Courbot M, Jacob C, Blaudez D, Chalot M (2006) Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol Lett 254:173–181
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Blaudez D, Jacob C, Turnau K, Colpaert JV, Ahonen-Jonnarth U, Finlay R, Botton B, Chalot M (2000) Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol Res 104:1366–1371
Bojarczuk K, Karliński L, Hazubska-Przybył T, Kieliszewska-Rokicka B (2015) Influence of mycorrhizal inoculation on growth of micropropagated Populus × canescens lines in metal-contaminated soils. New For 46:195–215
Cairney J (2012) Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil Biol Biochem 47:198–208
Chen S, Chen L, Ma Y, Huang Y (2009) Can phosphate compounds be used to reduce the plant uptake of Pb and resist the Pb stress in Pb-contaminated soils? J Environ Sci 21:360–365
Chen X, Wu C, Tang J, Hu S (2005) Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 60:665–671
Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S (2010) Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot 106:791–802
Corrêa A, Strasser MA, Martins-Loução (2006) Are mycorrhiza always beneficial? Plant Soil 279:65–73
Courty PE, Labbe J, Kohler A, Marcias B, Bastien C, Churin J, Garbaye J, Tacon F (2011) Effect of poplar genotypes on mycorrhizal infection and secreted enzyme activities in mycorrhizal and non-mycorrhizal roots. J Exp Bot 62:249–260
Danielsen L, Lohaus G, Sirrenberg A, Karlovsky P, Bastien C, Pilate G, Polle A (2013) Ectomycorrhizal colonization and diversity in relation to tree biomass and nutrition in a plantation of transgenic poplars with modified lignin biosynthesis. PLoS One 8(3):e59207
Fahr M, Laplaze L, Bendaou N, Hocher V, Mzibri ME, Bogusz D, Smouni A (2013) Effect of lead on root growth. Front Plant Sci 4:175
Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151:1991–2005
Felten J, Martin F, Legué V (2012) Signaling in ectomycorrhizal symbiosis. In: Baluška F, Vivanco J (eds) Signaling and Communication in Plants. Springer, Heidelberg Dordrecht London New York, pp 123–142
Gafur A, Schützendübel A, Langenfeld-Heyser R, Fritz E, Polle A (2004) Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus × canescens) differ in H2O2 production. Plant Biol (Stuttg) 6:91–99
Ijaz A, Imran A, Anwar ul Haq M, Khan Q, Afzal M (2016) Phytoremediation: recent advances in plant-endophytic synergistic interactions. Plant Soil 405:179–195
Jacob C, Courbot M, Martin F, Brun A, Chalot M (2004) Transcriptomic responses to cadmium in the ectomycorrhizal fungus Paxillus involutus. FEBS Lett 576:423–427
Jentschke G, Godbold DL (2000) Metal toxicity and ectomycorrhizas. Physiol Plan 109:107–116
Jiang W, Liu D (2010) Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol 10(40):1–8
Johansson EM, Fransson PAM, Finlay RD, van Hees PAV (2008) Quantitative analysis of root and ectomycorrhizal exudates as a response to Pb, Cd and As stress. Plant Soil 313:39–54
Jouve L, Hoffmann L, Hausman J-F (2004) Polyamine, carbohydrate, and proline content changes during salt stress exposure of Aspen (Populus tremula L.): involvement of oxidation and osmoregulation metabolism. Plant Biol (Stuttg) 6:74–80
Karliński L, Rudawska M, Leski T (2013) The influence of host genotype and soil conditions on ectomycorrhizal community of poplar clones. Eur J Soil Biol 58:51–58
Kopittke PM, Asher CJ, Menzies NW (2008) Prediction of Pb speciation in concentrated and dilute nutrient solutions. Environ Pollut 153:548–554
Kottke I, Guttenberger M, Hampp R, Oberwinkler F (1987) An in vitro method for establishing mycorrhizae on coniferous tree seedlings. Trees 1:191–194
Krause K, Henke C, Asiimwe T, Ulbricht A, Klemmer S, Schachtschabel D, Boland W, Kothe E (2015) Biosynthesis and secretion of indole-3-acetic acid and its morphological effects on Tricholoma vaccinum-spruce ectomycorrhiza. Appl Environ Microbiol 81:7003–7011
Krpata D, Peintner U, Langer I, Fitz WJ, Schweiger P (2008) Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol Res 112:1069–1079
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and role in defense strategy. Acta Physiol Plant 33:35–51
Lichtenthaler HK, Wellburn RR (1983) Determination of total carotenoids and chlorophylls a and b of extracts in different solvents. Biochem Soc Trans 603:591–592
Lorenc-Plucińska G, Walentynowicz M, Niewiadomska A (2013) Capabilities of alders (Alnus incana and A. glutinosa) to grow in metal-contaminated soil. Ecol Eng 58:214–227
Ma Y, He J, Ma C, Luo J, Li H, Liu T, Polle A, Peng C, Luo ZB (2014) Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ 37:627–642
Magrisso S, Belkin S, Erel Y (2009) Lead bioavailability in soil and soil components. Water Air Soil Pollut 202:315–323
Maltz MR, Treseder KT (2015) Sources of inocula influence mycorrhizal colonization of plants in restoration projects: a meta analysis. Restor Ecol 23:625–634
Marjanović Z, Uehlein N, Kaldenhoff R, Zwiazek JJ, Weiss M, Hampp R, Nehls U (2005) Aquaporins in poplar: what a difference a symbiont makes! Planta 222:258–268
Marschner P, Jentschke G, Godbold D (1998) Cation exchange capacity and lead sorption in ectomycorrhizal fungi. Plant Soil 205:93–98
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Mrnka L, Kuchár M, Cieslarová Z, Matějka P, Száková J, Tlustoš P, Vosátka M (2012) Effects of endo- and ectomycorrhizal fungi on physiological parameters and heavy metals accumulation of two species from the family Salicaceae. Water Air Soil Pollut 223:399–410
Münzenberger B, Kottke I, Oberwinkler F (1995) Reduction of phenolics in mycorrhizas of Larix decidua Mill. Tree Physiol 15:191–196
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Puigdomenech I. Medusa software, KTH University, Sweden). Available free from https://www.kth.se/en/che/medusa/downloads-1.386254
Rabęda I, Bilski H, Mellerowicz E, Napieralska A, Suski S, Woźny A, Krzesłowska M (2015) Colocalization of low-methylesterified pectins and Pb deposits in the apoplast of aspen roots exposed to lead. Environ Pollut 205:315–326
Rascio N, Flavia Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Rodríguez VM, Soengas P, Alonso-Villaverde V, Sotelo T, Cartea ME, Velasco P (2015) Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol 15:145
Rudawska ML, Kieliszewska-Rokicka B (1997) Mycorrhizal formation by Paxillus involutus strains in relation to their IAA-synthesizing activity. New Phytol 137:509–517
Sarjala T, Niemi K, Häggman H (2010) Mycorrhiza formation is not needed for early growth induction and growth-related changes in polyamines in Scots pine seedlings in vitro. Plant Physiol Biochem 48:596–601
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Smith SE, Read D (2008) Mycorrhizal symbiosis. Academic, London, UK
Sutela S, Niemi K, Edesi J, Laakso T, Saranpaa P, Vuosku J, Makela R, Tiimonen H, Chiang V, Koskimaki J, Suorsa M, Julkunen-Tiitto R, Haggman H (2009) Phenolic compounds in ectomycorrhizal interaction of lignin modified silver birch. BMC Plant Biol 9:124
Szuba A (2015) Ectomycorrhiza of Populus. For Ecol Manag 347:156–169
Szuba A, Lorenc-Plucińska G (2015) Utilization of proteomics in experimental field conditions—a case study of poplars growing on grassland affected by long-term starch wastewater irrigation. J Proteomics 126:200–217
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 939161:1–30
Tschaplinski TJ, Plett JM, Engle NL, Deveau A, Cushman KC, Martin MZ, Doktycz MJ, Tuskan GA, Brun A, Kohler A, Martin F (2014) Populus trichocarpa and Populus deltoids exhibit different metabolomic responses to colonization by the symbiotic fungus Laccaria bicolor. Mol Plant Microbe Interact 27:546–556
Turgeman T, Asher JB, Roth-Bejerano N, Kagan-Zur V, Kapulnik Y, Sitrit Y (2011) Mycorrhizal association between the desert truffle Terfezia boudieri and Helianthemum sessiliflorum alters plant physiology and fitness to arid conditions. Mycorrhiza 21:623–630
Turnau K, Orlowska E, Ryszka P, Zubek S, Anielska T, Gawronski S, Jurkiewicz A (2006) Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in Southern Poland. In: Twardowska I, Allen HE, Häggblom MM (eds) Soil and water pollution monitoring, protection and remediation. Springer, Dordrecht, pp 533–551
Vodnik D, Bozic M, Gogala N, Gabrovsek K (1996) Growth response of ectomycorrhizal Norway Spruce seedlings transplanted on lead-polluted soil. Phyton 36:77–80
Vodnik D, Byrne AR, Gogala N (1998) The uptake and transport of lead in some ectomycorrhizal fungi in culture. Mycol Res 102:953–958
Wang CR, Tian Y, Wang XR, Yu HX, Lu XW, Wang C, Wang H (2010) Hormesis effects and implicative application in assessment of lead-contaminated soils in roots of Vicia faba seedlings. Chemosphere 80:965–971
Willmann A, Thomfohrde S, Haensch R, Nehls U (2014) The poplar NRT2 gene family of high affinity nitrate importers: Impact of nitrogen nutrition and ectomycorrhiza formation. Environ Exp Bot 108:79–88
Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M (2015) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One 10(12):e0145726
Zarb J, Walters DDR (1996) Polyamine biosynthesis in the ectomycorrhizal fungus Paxillus involutus exposed to lead. Mycol Res 100:486–488
Zhao FJ, McGrath SP (2009) Biofortification and phytoremediation. Curr Opin Plant Biol 12:373–380
Acknowledgments
This work was funded by the National Science Centre, Poland (DEC-2011/03/D/NZ9/05500). The authors thank Prof. K. Bojarczuk, Prof. G. Lorenc-Plucińska, Prof. M. Rudawska, Asst. Prof. T. Leski, and Dr. J. Mucha from the Institute of Dendrology and Dr. Ł. Marczak from the Institute of Bioorganic Chemistry, Polish Academy of Sciences for their valuable assistance and discussions as well as Prof. M. Frankowski from the Faculty of Chemistry, Adam Mickiewicz University (Poznań, Poland) for help in predicting the solubility of Pb compounds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Fangjie Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 719 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Szuba, A., Karliński, L., Krzesłowska, M. et al. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant Soil 412, 253–266 (2017). https://doi.org/10.1007/s11104-016-3062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3062-3