Abstract
For Matthiola incana (Brassicaceae), used as a model system to study biochemical and genetical aspects of anthocyanin biosynthesis, several nearly isogenic colored wild type lines and white-flowering mutant lines are available, each with a specific defect in the genes responsible for anthocyanin production (genes e, f, and g). For gene f supposed to code for chalcone synthase (CHS; EC 2.3.1.74), the key enzyme of the flavonoid/anthocyanin biosynthesis pathway belonging to the group of type III polyketide synthases (PKS), the wild type genomic sequence of M. incana line 04 was determined in comparison to the white-flowering CHS mutant line 18. The type of mutation in the chs gene was characterized as a single nucleotide substitution in a triplet AGG coding for an evolutionary conserved arginine into AGT coding for serine (R72S). Northern blots and RT-PCR demonstrated that the mutated gene is expressed in flower petals. Heterologous expression of the wild type and mutated CHS cDNA in E. Scherichia coli, verified by Western blotting and enzyme assays with various starter molecules, revealed that the mutant protein had no detectable activity, indicating that the strictly conserved arginine residue is essential for the enzymatic reaction. This mutation, which previously was not detected by mutagenic screening, is discussed in the light of structural and functional information on alfalfa CHS and related type III PKS enzymes.
Similar content being viewed by others
References
Abe, I., Sano, Y., Takahashi, Y. and Noguchi, H. 2003. Sitedirected mutagenesis of benzalacetone synthase: the role of phe215 in plant type III polyketide synthases. JBC 278(27): 25218–25226.
Austin, M. B. and Noel, J. P. 2003. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 20: 79–120.
Austin, M. B., Bowman, M. E., Ferrer, J., Schröder, J. and Noel, J. P. An aldol switch discovered in stilbene synthases mediates aldol cyclization specificity of type III polyketides synthases. Chem. Biol. (in press).
Batschauer, A., Ehmann, B. and Schäfer, E. 1991. Cloning and characterization of a chalcone synthase gene from mustard and its light-dependent expression. Plant Mol. Biol. 16: 175–185.
Chappel, J. and Hahlbrock, K. 1984. Transcription of plant defence genes in response to UV-light or fungal elicitor. Nature 311: 76–78.
Dixon, R. A. and Steele, C. L. 1999. Flavonoids and isoflavonoids–a gold mine for metabolic genetic engineering. Trends Plant Sci. 4: 394–400.
Dooner, H. K., Robbins, T. P. and Jorgensen, R. A. 1991. Genetic and developmental control of anthocyanin biosynthesis. Annu. Rev. Genet. 25: 173–199.
Durbin, M. L., McCaig, B. and Clegg, M. T. 2000. Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol. Biol. 42: 79–92.
Epping, B., Kittel, M., Ruhnau, B. and Hemleben, V. 1990. Isolation and sequence analysis of a chalcone synthase cDNA of Matthiola incana R. Br. (Brassicaceae). Plant Mol. Biol. 14: 1061–1064.
Feinbaum, R. L. and Ausubel, F. M. 1988. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol. Cell Biol. 8: 1985–1992.
Ferrer, J.-L., Jez, J. M., Bowman, M. E., Dixon, R. A. and Noel, J. P. 1999. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nature Struct. Biol. 6: 775–783.
Forkmann, G. and Heller, W. 1999. Biosynthesis of flavonoids. In: S. Barton, O. Meth-Cohn (Eds.) Comprehensive Natural Products Chemistry, vol. 1, pp.713–748.
Forkmann, G. and Martens, S. 2001. Metabolic engineering and applications of flavonoids. Curr. Opin. Biotechnol. 12: 155–160.
Gebhardt, C., Ritter, E., Debener, T., Schachtschabel, U., Walkemeier, B., Uhrig, H. and Salamini, F. 1989. RFLP analysis and linkage mapping in Solanum tuberosum. Theor. Appl. Genet. 78: 65–75.
Hartmann, U., Valentine, W. J., Christie, J. M., Hays, J., Jenkins, G. I. and Weisshaar, B. 1998. Identification of UV/ blue-light response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system. Plant Mol. Biol. 36: 741–754.
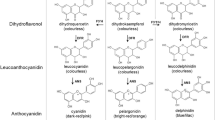
Heller, W., Britsch, L., Forkmann, G. and Grisebach, H. 1985. Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana. Planta 163: 191–196.
Hemleben V., Frey, M., Rall, S., Koch, M., Kittel, M., Kreuzaler, F., Ragg, H., Fautz, E. and Hahlbrock, K. 1982. Studies on the chalcone synthase gene of two higher plants: Petroselinum hortense and Matthiola incana. In: M. M. Burger, R. Weber, (Eds.) Embryonic Development. Part B: Cellular Aspects. ARL, New York pp. 555–566.
Hirner, A. A. and Seitz, H. U. 2000. Isoforms of chalcone synthase in Daucus carota L. and their differential expression in organs from the European wild carrot and in ultraviolet-Airradiated cell cultures. Planta 210: 993–998.
Jez, J. M. and Noel, J. P. 2000. Mechanisms of chalcone synthase: pKa of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J. Biol. Chem. 275: 39640–39646.
Jez, J. M., Austin, M. B., Ferrer, J.-L., Bowman, M. E., Schröder, J. and Noel, J. P. 2000a. Structural control of polyketide formation in plant-specific polyketide synthases. Chem. Biol. 7: 919–930.
Jez, J. M., Ferrer, J.-L., Bowman, M. E., Dixon, R. A. and Noel, J. P. 2000b. Dissection of malonyl-CoA decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39: 890–902.
Jez, J. M., Ferrer, J.-L., Bowman, M. E., Austin, M. B., Schröder, J., Dixon, R. A. and Noel, J. P. 2001. Structure and mechanism of chalcone synthase-like polyketide synthases. J. Indust. Microbiol. Biotechnol. 27: 393–398.
Kappert, H. 1949. Die Genetik des incana-Charakters und der Anthozyanbildung bei der Levkoje. Der Züchter 19: 289–297.
Kay, R., Chan, A., Damy, M. and McPherson, J. 1987. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299–1302.
Kessler, S. W. 1981. Use of protein A-bearing staphylococci for the immune precipitation and isolation of antigens from cells. Meth. Enzymol. 73: 442–459.
Koch, M. A., Weisshaar, B., Kroymann, J., Haubold, B. and Mitchell-Olds, T. 2001. Comparative genomics and regulatory evolution: conservation and function of the Chs and Apetala3 promoters. Mol. Biol. Evo. 18: 1882–1891.
Kowalczyk, E., Krzesinski, P., Kura, M., Szmigiel, B. and Blaszczyk, J. 2003. Anthocyanins in medicine. Pol. J. Pharmacol. 55: 699–702.
Lämmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Larkin, J. C., Oppenheimer, D. G., Lloyd, A. M., Paparozzi and Marks, M. D. 1994. Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell 6:1065–1076.
Lukačin, R., Springob, K., Urbanke, C., Ernwein, C., Schröder, G., Schröder, J. and Matern, U. 1999. Native acridone synthases I and II from Ruta graveolens form homodimers. FEBS Lett. 448: 135–140.
Lukačin, R., Gröning, I., Schiltz, E., Britsch, L. and Matern, U. 2000. Purification of recombinant flavanone 3b-hydroxylase from Petunia hybrida and assignment of the primary site of proteolytic degradation. Arch. Biochem. Biophys. 375: 364–370.
Lukačin, R., Schreiner, S. and Matern, U. 2001. Transformation of acridone synthase to chalcone synthase. FEBS Lett. 508: 413–417.
Lloyd, A. M., Walbot, V. and Davis, R. W. 1999. Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258: 1773–1775.
Martin, C. R. 1993. Structure, function, and regulation of the chalcone synthase. Int. Rev. Cytology, 147: 233–284.
Mettenleiter, T., Lukacs, N. and Rhiza, H. J. 1985. Mapping of the structural gene of Pseudorhabies Virus glycoprotein A and identification of two non-glycosylated precursor polypeptides. J. Virol. 53: 52–57.
Moore, B. S. and Hopke, J. N. 2001. Discovery of a new bacterial polyketide biosynthetic pathway. Chem. Bio. Chem. 2: 35–38.
Niesbach-Klösgen, U., Barzen, E., Bernhardt, J., Rhode, W., Schwarz-Sommer, Z., Reif, H. J., Wienand, U. and Saedler, H. 1987. Chalcone synthase genes in plants: a tool to study evolutionary relationships. J. Mol. Evo. 26: 213–225.
Rall, S. and Hemleben, V. 1984. Characterization and expression of chalcone synthase in different genotypes of Matthiola incana. Plant. Mol. Biol. 3: 137–145.
Ramsay, N. A., Walker, A. R., Mooney, M. and Gray, G. C. 2003. Two basic-loop-helix genes (MYC-146 and GL3 ) from Arabidopsis can activate anthocyanin biosynthesis in a 464 white-flowered Matthiola incana mutant. Plant Mol. Biol. 52: 679–688.
Rausher, M. D., Miller, R. E. and Tiffin, TP. 1999. Patterns of evolutionary rate variation among genes of the anthocyanin biosynthetic pathway. Mol. Biol. Evol. 16: 266–274.
Reimold, U., Kröger, M., Kreuzaler, F. and Hahlbrock, K. 1983. Coding and 3' non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 2: 1801–1805.
Saghai-Maroof, M. A., Soliman, K. M., Jorgensen, R. A. and Allard, R. W. 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81: 8014–8018.
Sali, A. and Blundell, T. L. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234: 779–815.
Sandermann, H. and Strominger, L. 1976. Purification and properties of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. J. Biol. Chem. 247: 5123–5131.
Saslowsky, D. E., Dana, D. D. and Winkel-Shirley, B. 2000. An allelic series for the chalcone synthase locus in Arabidopsis. Gene 255: 127–138.
Schröder, G., Brown, J. W. S. and Schröder J. 1988. Molecular analysis of resveratrol synthase: cDNA, genomic clones and relationship with chalcone synthase. Eur. J. Biochem. 172: 161–169.
Schröder, J. 1997. Afamily of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 2: 373–378.
Seyffert, W. 1971. Simulation of quantitative characters by genes with biochemically definable action. II. The material. Theor. Appl. Genet. 41: 285–291.
Seyffert, W. 1982. Beiträge zur Genetik und Enzymologie der Flavonoide. Biol. Zbl. 101: 465–483.
Spribille, R. and Forkmann, G. 1981. Genetic control of chalcone synthase activity in flowers of Matthiola incana R. Br. Z. Naturforschg. 36c: 619–624.
Springob, K., Nakajima J., Yamazaki, M. and Saito, K. 2003. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 20: 288–303.
Szymanski, D. B., Lloyd, A. M. and Marks M. D. 2000. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends Plant Sci. 5: 214–219.
Thompson, J. D., Higgins, D. G. and Gibson, T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680.
Towbin, H., Staehlin, T. and Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354.
Tropf, S., Kärcher, B., Schröder, G. and Schröder, J. 1995. Reaction mechanisms of homodimeric plant polyketide synthases (stilbene and chalcone synthases): a single active site for the condensing reaction is sufficient for synthesis of stilbenes, chalcones, and 6'-deoxychalcones. J Biol. Chem. 270: 7922–7928.
Walker, A. R., Davison, P. A., Bolognesi-Winfield, A. C., James, C. M. and Srinivasan, N. 1999. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 11: 1337–1350.
Weisshaar, B., Armstrong, G. A., Block, A., Costa e Silva O. and Hahlbrock K. 1991. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 10: 1777–1786.
Yamazaki, Y., Suh, D. Y., Sitthithaworn, W., Ishiguro, K., Kobayashi, Y., Shibuya, M., Ebizuka, Y. and Sankawa, U. 2001. Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta 214: 75–84.
Zheng, D., Schröder, G., Schröder, J. and Hrazdina, G. 2001. Molecular and biochemical characterization of three aromatic polyketide synthase genes from Rubus idaeus. Plant Mol. Biol. 46: 1–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hemleben, V., Dressel, A., Epping, B. et al. Characterization and structural features of a chalcone synthase mutation in a white-flowering line of Matthiola incanaR. Br. (Brassicaceae). Plant Mol Biol 55, 455–465 (2004). https://doi.org/10.1007/s11103-004-1125-y
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-1125-y