Abstract
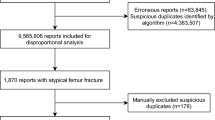
Background Signal generation through data mining algorithms is an innovative and emerging field in pharmacovigilance. Early detection of safety signals is important for public health safety. However, the possibility of generating pseudo signals should not be overlooked. Objective Our study aimed to identify potential signals of aromatase inhibitors associated Osteonecrosis of Jaw and assess the possibilities of the safety signal to be a pseudo signal/false positive in FDA Adverse Event Reporting System (FAERS). Setting Spontaneously reported data in FAERS database. Methods Data for this study were obtained from the public release of data in FAERS. OpenVigil, a pharmacovigilance analytical tool was used to access FAERS data. Reporting Odds Ratio (ROR) was used to assess the relation between the drug and adverse event. A value of ROR-1.96SE > 1, (SE—standard error) was considered positive. Main outcome measure Signal strength. Results FAERS database had a total of 15,178 reports for Osteonecrosis of Jaw. Amongst which 617 reports were associated with aromatase inhibitors. Signal strength ROR (lower bound of the 95% CI) for letrozole, anastrozole and exemestane associated Osteonecrosis of Jaw without any background correction was 8.34, 6.64 and 15.14 respectively. Upon removing the reports of concomitantly administered drugs (bisphosphonates and denosumab), signal strength drastically decreased to 0.03, 0.36 and 0.47 for letrozole, anastrozole and exemestane respectively. The signal strength of bisphosphonates and denosumab associated Osteonecrosis of Jaw was not changed significantly upon removal of aromatase inhibitors. Conclusion Our study concluded that the signal generated for aromatase inhibitors associated Osteonecrosis of Jaw in FAERS database can be false positive. Careful background corrections with identification of those risk factors are imperative to exclude false positive results.




Similar content being viewed by others
References
Kennedy DL, Goldman SA, Lillie RB. Spontaneous reporting in the United States. Pharmacoepidemiology, 3 ed. England: Wiley; 2000. p. 149–74.
Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36(2):75–81.
Organization WH. Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. Geneva: World Health Organization; 2002.
Meyboom RH, Egberts AC, Edwards IR, Hekster YA, de Koning FH, Gribnau FW. Principles of signal detection in pharmacovigilance. Drug Saf. 1997;16(6):355–65.
Härmark L, Van Grootheest A. Pharmacovigilance: methods, recent developments and future perspectives. Eur J Clin Pharmacol. 2008;64(8):743–52.
Lindquist M, Ståhl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000;23(6):533–42.
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57(2):127–34.
Hauben M, Zhou X. Quantitative methods in pharmacovigilance. Drug Saf. 2003;26(3):159–86.
Almenoff J, Tonning JM, Gould AL, Szarfman A, Hauben M, Ouellet-Hellstrom R, et al. Perspectives on the use of data mining in pharmacovigilance. Drug Saf. 2005;28(11):981–1007.
Ahmed I, Dalmasso C, Haramburu F, Thiessard F, Broët P, Tubert-Bitter P. False discovery rate estimation for frequentist pharmacovigilance signal detection methods. Biometrics. 2010;66(1):301–9.
FDA Adverse Event Reporting System (FAERS) Public Dashboard FDA. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/ucm070093.htm. Accessed 27 July 2019.
Böhm R, von Hehn L, Herdegen T, Klein H-J, Bruhn O, Petri H, et al. OpenVigil FDA—inspection of US American adverse drug events pharmacovigilance data and novel clinical applications. PLoS ONE. 2016;11(6):e0157753.
Poluzzi E, Raschi E, Piccinni C, De Ponti F (2012) Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA adverse event reporting system (AERS). In: Data mining applications in engineering and medicine, inTech.
Subeesh V, Singh H, Maheswari E, Beulah E. Novel adverse events of vortioxetine: a disproportionality analysis in USFDA adverse event reporting system database. Asian Journal of Psychiatry. 2017;30:152–6.
FDAble http://www.fdable.com/basic_query/aers_analysis. Accessed 4 Aug 2019.
Pratt LA, Danese PN. More eyeballs on AERS. Nat Biotechnol. 2009;27(7):601.
Grigoriev I, zu Castell W, Tsvetkov P, Antonov AV. AERS spider: an online interactive tool to mine statistical associations in adverse event reporting system. Pharmacoepidemiol Drug Saf. 2014;23(8):795–801.
Hauben M, Madigan D, Gerrits CM, Walsh L, Van Puijenbroek EP. The role of data mining in pharmacovigilance. Exp Opin Drug Saf. 2005;4(5):929–48.
Hamadeh IS, Ngwa BA, Gong Y. Drug induced osteonecrosis of the jaw. Cancer Treat Rev. 2015;41(5):455–64.
Gupta S, Gupta H, Mandhyan D, Srivastava S. Bisphophonates related osteonecrosis of the jaw. Natl J Maxillofac Surg. 2013;4(2):151.
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56.
Nicolatou-Galitis O, Schiødt M, Mendes RA, Ripamonti C, Hope S, Drudge-Coates L, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117–35.
Acknowledgements
We thank Ms. Radhika, Department of community medicine, Ramaiah Medical College, Bengaluru, India, who clarified all the statistical related doubts. We thank our Dean & Professors, Faculty of Pharmacy, M S Ramaiah University of Applied Sciences who provided insight and expertise that greatly assisted the research. We thank Dr.Ruwen Böhm, Institute of Experimental and Clinical Pharmacology, University Hospital Schleswig–Holstein, Campus Kiel, Kiel, Germany, for assistance with open VigilFDA v1.0.2 that greatly improved the manuscript.
Funding
No funding received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neha, R., Beulah, E., Anusha, B. et al. Aromatase inhibitors associated osteonecrosis of jaw: signal refining to identify pseudo safety signals. Int J Clin Pharm 42, 721–727 (2020). https://doi.org/10.1007/s11096-020-01018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-020-01018-z