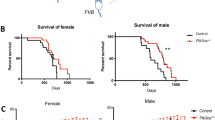
Abstract
Purpose
Individuals with the rare genetic disorder Pitt Hopkins Syndrome (PTHS) do not have sufficient expression of the transcription factor 4 (TCF4) which is located on chromosome 18. TCF4 is a basic helix-loop-helix E protein that is critical for the normal development of the nervous system and the brain in humans. PTHS patients lacking sufficient TCF4 frequently display gastrointestinal issues, intellectual disability and breathing problems. PTHS patients also commonly do not speak and display distinctive facial features and seizures. Recent research has proposed that decreased TCF4 expression can lead to the increased translation of the sodium channel Nav1.8. This in turn results in increased after-hyperpolarization as well as altered firing properties. We have recently identified through a drug repurposing screen an FDA approved dihydropyridine calcium antagonist nicardipine used to treat angina, which inhibited Nav1.8.
Methods
We have now performed behavioral testing in groups of 10 male Tcf4(± ) PTHS mice dosing by oral gavage at 3 mg/kg once a day for 3 weeks using standard methods to assess sociability, nesting, fear conditioning, self-grooming, open field and test of force.
Results
Nicardipine returned this spectrum of behavioral deficits in the Tcf4(± ) PTHS mouse model to WT levels and resulted in statistically significant results.
Conclusions
These in vivo results in the well characterized Tcf4(± ) PTHS mice may suggest the potential to test this already approved drug further in a clinical study with PTHS patients or suggest the potential for use off label under compassionate use with their physician.






Similar content being viewed by others
References
Ekins S, Wood J. Incentives for starting small companies focused on rare and neglected diseases. Pharm Res. 2016;33(4):809–15.
Dalecki AG, Zorn KM, Clark AM, Ekins S, Narmore WT, Tower N, et al. High-throughput screening and Bayesian machine learning for copper-dependent inhibitors of Staphylococcus aureus. Metallomics. 2019;11(3):696–706.
Ekins S, Williams AJ, Krasowski MD, Freundlich JS. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today. 2011;16(7–8):298–310.
Baker NC, Ekins S, Williams AJ, Tropsha A. A bibliometric review of drug repurposing. Drug Discov Today. 2018;23(3):661–72.
Delavan B, Roberts R, Huang R, Bao W, Tong W, Liu Z. Computational drug repositioning for rare diseases in the era of precision medicine. Drug Discov Today. 2018;23(2):382–94.
Valencic E, Smid A, Jakopin Z, Tommasini A, Mlinaric-Rascan I. Repositioning drugs for rare immune diseases: hopes and challenges for a precision medicine. Curr Med Chem. 2018;25(24):2764–82.
Pitt D, Hopkins I. A syndrome of mental retardation, wide mouth and intermittent overbreathing. Aust Paediatr J. 1978;14(3):182–4.
Brockschmidt A, Todt U, Ryu S, Hoischen A, Landwehr C, Birnbaum S, et al. Severe mental retardation with breathing abnormalities (Pitt-Hopkins syndrome) is caused by haploinsufficiency of the neuronal bHLH transcription factor TCF4. Hum Mol Genet. 2007;16(12):1488–94.
Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome). Am J Hum Genet. 2007;80(5):994–1001.
Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80(5):988–93.
Zollino M, Zweier C, Van Balkom ID, Sweetser DA, Alaimo J, Bijlsma EK, et al. Diagnosis and management in Pitt-Hopkins syndrome: First international consensus statement. Clin Genet. 2019;95(4):462–78.
Li H, Zhu Y, Morozov YM, Chen X, Page SC, Rannals MD, et al. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol Psychiatry. 2019;24(8):1235–46.
Crux S, Herms J, Dorostkar MM. Tcf4 regulates dendritic spine density and morphology in the adult brain. PLoS One. 2018;13(6):e0199359.
Goodspeed K, Newsom C, Morris MA, Powell C, Evans P, Golla S. Pitt-Hopkins syndrome: a review of current literature, clinical approach, and 23-patient case series. J Child Neurol. 2018;33(3):233–44.
Mary L, Piton A, Schaefer E, Mattioli F, Nourisson E, Feger C, et al. Disease-causing variants in TCF4 are a frequent cause of intellectual disability: lessons from large-scale sequencing approaches in diagnosis. Eur J Hum Genet. 2018;26(7):996–1006.
Tan A, Goodspeed K, Edgar VB. Pitt-Hopkins syndrome: a unique case study. J Int Neuropsychol Soc. 2018;24(9):995–1002.
Kennedy AJ, Rahn EJ, Paulukaitis BS, Savell KE, Kordasiewicz HB, Wang J, et al. Tcf4 regulates synaptic plasticity, DNA methylation, and memory function. Cell Rep. 2016;16(10):2666–85.
Thaxton C, Kloth AD, Clark EP, Moy SS, Chitwood RA, Philpot BD. Common pathophysiology in multiple mouse models of Pitt-Hopkins syndrome. J Neurosci. 2018;38(4):918–36.
Rannals MD, Hamersky GR, Page SC, Campbell MN, Briley A, Gallo RA, et al. Psychiatric risk gene transcription factor 4 regulates intrinsic excitability of prefrontal neurons via repression of SCN10a and KCNQ1. Neuron. 2016;90(1):43–55.
Rannals MD, Page SC, Campbell MN, Gallo RA, Mayfield B, Maher BJ. Neurodevelopmental models of transcription factor 4 deficiency converge on a common ion channel as a potential therapeutic target for Pitt Hopkins syndrome. Rare Dis. 2016;4(1):e1220468.
Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA. Voltage gated sodium channels as drug discovery targets. Channels (Austin). 2015;9(6):360–6.
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–25.
Plummer NW, Meisler MH. Evolution and diversity of mammalian sodium channel genes. Genomics. 1999;57(2):323–31.
Rabert DK, Koch BD, Ilnicka M, Obernolte RA, Naylor SL, Herman RC, et al. A tetrodotoxin-resistant voltage-gated sodium channel from human dorsal root ganglia, hPN3/SCN10A. Pain. 1998;78(2):107–14.
Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379(6562):257–62.
Akopian ANSV, England S, Okuse K, Ogata N, Ure J, Smith A, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–8.
Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131(3):243–57.
Nardi A, Damann N, Hertrampf T, Kless A. Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem. 2012;7(10):1712–40.
Gurney ME, Cogram P, Deacon RM, Rex C, Tranfaglia M. Multiple behavior phenotypes of the fragile-X syndrome mouse model respond to chronic inhibition of Phosphodiesterase-4D (PDE4D). Sci Rep. 2017;7(1):14653.
Tranfaglia MR, Thibodeaux C, Mason DJ, Brown D, Roberts I, Smith R, et al. Repurposing available drugs for neurodevelopmental disorders: the fragile X experience. Neuropharmacology. 2019;147:74–86.
Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1(3):1117–9.
Doostparast Torshizi A, Armoskus C, Zhang H, Forrest MP, Zhang S, Souaiaia T, et al. Deconvolution of transcriptional networks identifies TCF4 as a master regulator in schizophrenia. Sci Adv. 2019;5(9):eaau4139.
Pedersen CS, Sorensen DB, Parachikova AI, Plath N. PCP-induced deficits in murine nest building activity: employment of an ethological rodent behavior to mimic negative-like symptoms of schizophrenia. Behav Brain Res. 2014;273:63–72.
Tartaglione AM, Armida M, Potenza RL, Pezzola A, Popoli P, Calamandrei G. Aberrant self-grooming as early marker of motor dysfunction in a rat model of Huntington's disease. Behav Brain Res. 2016;313:53–7.
Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158(6):1348–61.
Hegyi B, Komaromi I, Nanasi PP, Szentandrassy N. Selectivity problems with drugs acting on cardiac Na(+) and Ca(2)(+) channels. Curr Med Chem. 2013;20(20):2552–71.
Langle D, Marquardt V, Heider E, Vigante B, Duburs G, Luntena I, et al. Design, synthesis and 3D-QSAR studies of novel 1,4-dihydropyridines as TGFbeta/Smad inhibitors. Eur J Med Chem. 2015;95:249–66.
Czuczwar SJ, Gasior M, Janusz W, Kleinrok Z. Influence of flunarizine, nicardipine and nimodipine on the anticonvulsant activity of different antiepileptic drugs in mice. Neuropharmacology. 1992;31(11):1179–83.
Whiting RL. Animal pharmacology of nicardipine and its clinical relevance. Am J Cardiol. 1987;59(17):3J–8J.
Michel AD, Whiting RL. Cellular action of nicardipine. Am J Cardiol. 1989;64(15):3H–7H.
Huang BR, Chang PC, Yeh WL, Lee CH, Tsai CF, Lin C, et al. Anti-neuroinflammatory effects of the calcium channel blocker nicardipine on microglial cells: implications for neuroprotection. PLoS One. 2014;9(3):e91167.
Bachmeier C, Beaulieu-Abdelahad D, Mullan M, Paris D. Selective dihydropyiridine compounds facilitate the clearance of beta-amyloid across the blood-brain barrier. Eur J Pharmacol. 2011;659(2–3):124–9.
Anon. Nicardipine Hydrochloride Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=b8f147b8-955f-4733-9178-2cfa0beb60d6&type=display.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31.
ACKNOWLEDGMENTS AND DISCLOSURES
These studies were supported by a grant from the Pitt-Hopkins Research Foundation. Dr’s Patricia Cogram, Robert M.J. Deacon and Daniel Benitez are sincerely acknowledged for generating the data in this study under a contract with the Pitt-Hopkins Research Foundation. Special thanks are also due to Dr. Michael Tranfaglia for his intellectual support to Dr. Cogram and Dr. Deacon throughout the design and implementation of these experiments. The authors wish to thank Dr. Andrew J. Kennedy from the University of Alabama at Birmingham for kindly sending us the mice and the families of the Pitt-Hopkins Research Foundation for financial and moral support at the earliest stages of initiating these studies. SE acknowledges the support and discussions with Dr’s Aaron Gerlach, Aaron McMurtray and Kimberly Goodspeed. ACP kindly acknowledges funding from NIH/ NIAID 3R43NS107079-01S1. SE is CEO and Founder of Collaborations Pharmaceuticals, Inc., and has submitted a provisional patent and orphan drug designation on nicardipine for Pitt Hopkins Syndrome. AP is an employee at Collaborations Pharmaceuticals, Inc. AD is President of the Pitt Hopkins Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekins, S., Puhl, A.C. & Davidow, A. Repurposing the Dihydropyridine Calcium Channel Inhibitor Nicardipine as a Nav1.8 Inhibitor In Vivo for Pitt Hopkins Syndrome. Pharm Res 37, 127 (2020). https://doi.org/10.1007/s11095-020-02853-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02853-5