Abstract
Purpose
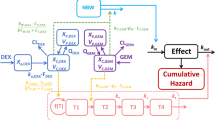
Paclitaxel (PTX)-loaded genipin-crosslinked gelatin microspheres (GP-MS) are a prolonged IP delivery system under development for the treatment of peritoneal minimal residual disease (pMRD). Here, we show the use of a pharmacokinetic-pharmacodynamic (PKPD) modelling approach to inform the formulation development of PTX-GP-MS in a mice pMRD model.
Methods
PTX blood concentrations and survival data were obtained in Balb/c Nu mice receiving different single IP doses (7.5 and/or 35 mg/kg) of PTX-ethanolic loaded GP-MS (PTXEtOH-GP-MS), PTX-nanosuspension loaded GP-MS (PTXnano-GP-MS), and immediate release formulation Abraxane®. A population PK model was developed to characterize the PTX blood concentration pattern and to predict PTX concentrations in peritoneum. Afterwards, PKPD relationships between the predicted peritoneal or blood concentrations and survival were explored using time-to-event modelling.
Results
A PKPD model was developed that simultaneously describes the competing effects of treatment efficacy (driven by peritoneal concentration) and toxicity (driven by blood concentration) of PTX on survival. Clear survival advantages of PTXnano-GP-MS over PTXEtOH-GP-MS and Abraxane® were found. Simulations of different doses of PTXnano-GP-MS demonstrated that drug-induced toxicity is high at doses between 20 and 35 mg/kg.
Conclusions
The model predicts that the dose range of 7.5-15 mg/kg of PTXnano-GP-MS provides an optimal balance between efficacy and safety.





Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike’s information criterion
- BLI:
-
Bioluminescence imaging
- CRS:
-
Cytoreductive surgery
- DBS:
-
Dried blood spot
- GOF:
-
Goodness of fit
- GP-MS:
-
Genipin-crosslinked gelatin microspheres
- IIV:
-
Inter-individual variability
- IPC:
-
Intraperitoneal chemotherapy
- LLOQ:
-
Lower limit of quantification
- Nab-PTX:
-
Paclitaxel-loaded albumin nanoparticles
- OFV:
-
Objective function value
- pcVPCs:
-
Prediction-corrected visual predictive checks
- PKPD:
-
Pharmacokinetic-pharmacodynamic
- pMRD:
-
Peritoneal minimal residual disease
- PTX:
-
Paclitaxel
- PTX-GP-MS:
-
Paclitaxel-loaded genipin-crosslinked gelatin microspheres
- PTXEtOH-GP-MS:
-
Paclitaxel-ethanolic loaded genipin-crosslinked gelatin microspheres
- PTXnano-GP-MS:
-
Paclitaxel-ethanolic loaded genipin-crosslinked gelatin microspheres
- SIR:
-
Sampling importance resampling
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Nosov V, Su F, Amneus M, Birrer M, Robins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009;200(6):639. e631–9.
Tummala MK, Alagarsamy S, McGuire WP. Intraperitoneal chemotherapy: standard of care for patients with minimal residual stage III ovarian cancer? Expert Rev Anticancer Ther. 2008;8(7):1135–47.
Du Bois A. Treatment of advanced ovarian cancer. Eur J Cancer. 2001;37:1–7.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6.
Ozols R. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15:3–11.
Berrino F, De Angelis R, Sant M, Rosso S, Lasota MB, Coebergh JW, Santaquilani M, Group EW. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol 2007;8(9):773-783.
Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009;115(6):1234–44.
Chefetz I, Alvero A, Holmberg J, Lebowitz N, Craveiro V, Yang-Hartwich Y, et al. TLR2 enhances ovarian cancer stem cell self-renewal and promotes tumor repair and recurrence. Cell Cycle. 2013;12(3):511–21.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. nature. 2001;414(6859):105.
Bijelic L, Jonson A, Sugarbaker P. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007;18(12):1943–50.
Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009;135(12):1637–45.
Oršolić N, Bevanda M, Kujundžić N, Plazonic A, Stajcar D, Kujundžić M. Prevention of peritoneal carcinomatosis in mice by combining hyperthermal intraperitoneal chemotherapy with the water extract from Burr parsley (Caucalis platycarpos L.). Planta Med. 2010;76(08):773–9.
Bakrin N, Classe J, Pomel C, Gouy S, Chene G, Glehen O. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J Visc Surg. 2014;151(5):347–53.
De Bree E, Helm CW. Hyperthermic intraperitoneal chemotherapy in ovarian cancer: rationale and clinical data. Expert Rev Anticancer Ther. 2012;12(7):895–911.
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–7.
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43.
Van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HW, Hermans RH, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–40.
Spiliotis J, Vaxevanidou A, Sergouniotis F, Lambropoulou E, Datsis A, Christopoulou A. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of recurrent advanced ovarian cancer: a prospective study. J BUON. 2011;16(1):74–9.
Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis Jr JL, Rubin S, Curtin J, Barakat R, Phillips M. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol 1992;10(9):1485-1491.
Tsai M, Lu Z, Wientjes MG, Au JL-S. Paclitaxel-loaded polymeric microparticles: quantitative relationships between in vitro drug release rate and in vivo pharmacodynamics. J Control Release. 2013;172(3):737–44.
Vassileva V, Moriyama E, De Souza R, Grant J, Allen C, Wilson B, et al. Efficacy assessment of sustained intraperitoneal paclitaxel therapy in a murine model of ovarian cancer using bioluminescent imaging. Br J Cancer. 2008;99(12):2037.
De Clercq K, Schelfhout C, Bracke M, De Wever O, Van Bockstal M, Ceelen W, et al. Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal adhesions: In vitro and in vivo characterization. Biomaterials. 2016;96:33–46.
Xie F, De Thaye E, Vermeulen A, Van Bocxlaer J, Colin P. A dried blood spot assay for paclitaxel and its metabolites. J Pharm Biomed Anal. 2018;148:307–15.
Gao Y, Shen JK, Choy E, Zhang Z, Mankin HJ, Hornicek FJ, et al. Pharmacokinetics and tolerability of NSC23925b, a novel P-glycoprotein inhibitor: preclinical study in mice and rats. Sci Rep. 2016;6:25659.
Liu X-R, Wu K-C, Huang Y, Sun J-B, Ke X-Y, Wang J-C, et al. In vitro and in vivo studies on plasma-to-blood ratio of paclitaxel in human, rabbit and rat blood fractions. Biol Pharm Bull. 2008;31(6):1215–20.
Owen JS, Fiedler-Kelly J. Introduction to population pharmacokinetic/pharmacodynamic analysis with nonlinear mixed effects models: John Wiley & Sons; 2014.
Au JL-S, Guo P, Gao Y, Lu Z, Wientjes MG, Tsai M, et al. Multiscale tumor spatiokinetic model for intraperitoneal therapy. AAPS J. 2014;16(3):424–39.
Dosne A-G, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43(6):583–96.
Upton R, Mould D. Basic concepts in population modeling, simulation, and Model-Based drug development: Part 3—Introduction to pharmacodynamic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2014;3(1):1–16.
Schettini F, Giuliano M, De Placido S, Arpino G. Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives. Cancer Treat Rev. 2016;50:129–41.
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24.
Badr CE. Bioluminescence imaging: basics and practical limitations. In. Bioluminescent Imaging: Springer; 2014. p. 1-18.
Van Goor H. Consequences and complications of peritoneal adhesions. Color Dis. 2007;9:25–34.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a Concerted Research Action (GOA) grant (project number: 01G02916) from Ghent University. Feifan Xie acknowledges the China Scholarship Council (CSC) for his Ph.D. grant. An Vermeulen is an 80% employee of Johnson and Johnson and owns J&J stock/stock options. She is also a visiting Professor at Ghent University. All other authors declare have no conflicts of interest that are directly relevant to the contents of this manuscript. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. (Animal Ethics Committee of the Faculty of Medicine at Ghent University, permit number: ECD 17/83).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, F., De Clercq, K., Vervaet, C. et al. Model-based analysis of treatment effects of paclitaxel microspheres in a microscopic peritoneal carcinomatosis model in mice. Pharm Res 36, 127 (2019). https://doi.org/10.1007/s11095-019-2660-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2660-1