Abstract
Purpose
The renal clearance of fampridine (Fampyra®, or Ampyra®) significantly exceeds the glomerular filtration rate, suggesting active renal secretion is likely the major elimination pathway. The goal of this study was to identify the renal transporters that are involved in the renal active secretion, and elucidate the active renal secretion mechanism of fampridine.
Methods
The uptake of fampridine to HEK-293 cells overexpressing human OCT2, MATE1 or MATE2K was determined in the absence and presence of Cimetidine, the prototypical inhibitor of the transporters. The inhibition potential of fampridine on the renal transporters was evaluated by determining the uptake of TEA and Metformin, the probe substrates of the transporters of OCT2 and MATEs, respectively, in the absence or presence of fampridine.
Results
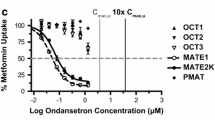
Significant time- and concentration-dependent uptake of fampridine by human OCT2 was observed. The Km and Vmax were determined as 51.0 ± 17.1 μM and 1107 ± 136 pmole/min/106 cells, respectively. Fampridine also inhibited OCT2 mediated uptake of Metformin with estimated IC50 of 66.8 μM. In contrast, there was not significant uptake of fampridine by human MATE1 or MATE2K, and fampridine did not inhibit MATE1 or MATE2K mediated uptake of TEA.
Conclusion
The studies indicated fampridine is a substrate and inhibitor of OCT2, but not MATE1 or MATE2K. Results from the study suggested the active renal secretion of fampridine is mediated by human OCT2 but not MATE1 or MATE2K. To our knowledge, fampridine is the first reported substrate specific to OCT2 but not to MATE1 or MATE2K.




Similar content being viewed by others
References
Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci. 2006;95(1):25–36.
Komatsu T, Hiasa M, Miyaji T, Kanamoto T, Matsumoto T, Otsuka M, et al. Characterization of the human MATE2 proton-coupled polyspecific organic cation exporter. Int J Biochem Cell Biol. 2011;43(6):913–8.
Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. American journal of physiology Renal physiology. 2010;298(4):F997–F1005.
Motohashi H, Nakao Y, Masuda S, Katsura T, Kamba T, Ogawa O, et al. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J Pharm Sci. 2013;102(9):3302–8.
Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal drug transporters and drug interactions. Clin Pharmacokinet. 2017;56:825–92.
Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol. 2013;273(1):100–9.
Chen J, Brockmoller J, Seitz T, Konig J, Tzvetkov MV, Chen X. Tropane alkaloids as substrates and inhibitors of human organic cation transporters of the SLC22 (OCT) and the SLC47 (MATE) families. Biol Chem. 2017;398(2):237–49.
Hibma JE, Zur AA, Castro RA, Wittwer MB, Keizer RJ, Yee SW, et al. The effect of famotidine, a MATE1-selective inhibitor, on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacokinet. 2016;55(6):711–21.
Morrissey KM, Stocker SL, Chen EC, Castro RA, Brett CM, Giacomini KM. The effect of Nizatidine, a MATE2K selective inhibitor, on the pharmacokinetics and pharmacodynamics of metformin in healthy volunteers. Clin Pharmacokinet. 2016;55(4):495–506.
Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011;54(13):4548–58.
Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2):359–71.
Reznicek J, Ceckova M, Cerveny L, Muller F, Staud F. Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp. BCRP or MRP2 transporters Xenobiotica; the fate of foreign compounds in biological systems. 2017;47(1):77–85.
Minematsu T, Giacomini KM. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther. 2011;10(3):531–9.
Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–67.
Motohashi H, Inui K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013;15(2):581–8.
Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013;56(3):781–95.
Ito S, Kusuhara H, Kuroiwa Y, Wu C, Moriyama Y, Inoue K, et al. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther. 2010;333(1):341–50.
Kato K, Moriyama C, Ito N, Zhang X, Hachiuma K, Hagima N, et al. Involvement of organic cation transporters in the clearance and milk secretion of thiamine in mice. Pharm Res. 2015;32(7):2192–204.
Hacker K, Maas R, Kornhuber J, Fromm MF, Zolk O. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One. 2015;10(9):e0136451.
Izumi S, Nozaki Y, Komori T, Maeda K, Takenaka O, Kusano K, et al. Substrate-Dependent Inhibition of Organic Anion Transporting Polypeptide 1B1: Comparative Analysis with Prototypical Probe Substrates Estradiol-17 beta-Glucuronide, Estrone-3-Sulfate, and Sulfobromophthaleins. Drug Metab Dispos 2013;41(10):1859–1866, Substrate-Dependent Inhibition of Organic Anion Transporting Polypeptide 1B1: Comparative Analysis with Prototypical Probe Substrates Estradiol-17 -Glucuronide, Estrone-3-Sulfate, and Sulfobromophthalein.
Noe J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35(8):1308–14.
Yin J, Duan HC, Wang JN. Impact of substrate-dependent inhibition on renal organic cation transporters hOCT2 and hMATE1/2-K-mediated drug transport and intracellular accumulation. J Pharmacol Exp Ther. 2016;359(3):401–10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, G., Rowbottom, C., Boiselle, C. et al. Fampridine is a Substrate and Inhibitor of Human OCT2, but not of Human MATE1, or MATE2K. Pharm Res 35, 159 (2018). https://doi.org/10.1007/s11095-018-2445-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2445-y