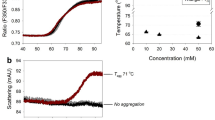
Abstract
Purpose
Understanding the mechanism of protein-excipient interaction and illuminating the influencing factors on protein stability are key steps in the rational design of protein formulations. The objective of this study was to assess effects of preferential interaction type of excipient and surface aromatic hydrophobicity of protein on protein solution stability.
Methods
The preferential interaction between excipient and aromatic hydrophobic area of protein was investigated by solubility and fluorescence studies of amino acid derivatives in excipient solutions. We examined conformational, colloidal and mechanical stabilities of model proteins with different surface aromatic hydrophobicities, including bovine serum albumin (BSA) and ovalbumin (OVA), and then stability data were visualized by three-index empirical phase diagram.
Results
The result showed that preferentially excluded excipients (trehalose, sucrose and sorbitol) protected protein conformation against damage, but they could accelerate mechanical stress-induced aggregation. Preferentially bound excipients (propanediol and arginine) suppressed BSA aggregation, but arginine failed to inhibit OVA aggregation, which might be attributed to the disparate conformational perturbing effects of arginine on aromatic hydrophobic regions of BSA and OVA.
Conclusions
These findings provided strong evidence that excipient possessed bilateral effects, and its application should be determined on different preferential interaction behaviors of excipients with protein, especially with the aromatic hydrophobic region.











Similar content being viewed by others
Abbreviations
- ANS:
-
8-Anilino-1-Naphthalenesulfonic acid
- BSA:
-
Bovine serum albumin
- DLS:
-
Dynamic light scattering
- EPD:
-
Empirical phase diagram
- FACS:
-
Flow cytometry
- FSC:
-
Forward scatter
- NAPA:
-
N-acetyl-L-phenylalanine amide
- NATA:
-
N-acetyl-L-tryptophanamide
- NAYA:
-
N-acetyl-L-tyrosinamide
- OD350nm :
-
Optical density at a wavelength of 350 nm
- OVA:
-
Ovalbumin
- SSC:
-
Side scatter
References
Micklus A, Muntner S. Deal watch: Biopharma deal-making in 2015: changing the pharma landscape. Nat Rev Drug Discov. 2016;15(2):78–9.
Topp EM. Commentary: current perspectives on the aggregation of protein drugs. AAPS J. 2014;16(3):413–4.
Rawat A, Burgess DJ. Parenteral Delivery of Peptideds and proteins. In: Morishita M, Park K, editors. Biodrug Delivery systems: fundamentals, applications and clinical development. New York: Informa Healthcare; 2009. p. 50–68.
Ezan E. Pharmacokinetic studies of protein drugs: past, present and future. Adv Drug Deliv Rev. 2013;65(8):1065–72.
Joubert MK, Hokom M, Eakin C. Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses. J Biol Chem. 2012;287(30):25266–79.
Chaudhuri R, Cheng Y, Middaugh CR, Volkin DB. High-throughput biophysical analysis of protein therapeutics to examine interrelationships between aggregate formation and conformational stability. AAPS J. 2014;16(1):48–64.
Telikepalli SN, Kumru OS, Kalonia C, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Structural characterization of IgG1 mAb aggregates and particles generated under various stress conditions. J Pharm Sci. 2014;103(3):796–809.
Kiese S, Papppenberger A, Friess W, Mahler HC. Shaken, not stirred: mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97(10):4347–66.
Wang W. Advanced protein formulations. Protein Sci. 2015;24(7):1031–9.
Hamrang Z, Rattray NJW, Pluen A. Proteins behaving badly: emerging technologies in profiling biopharmaceutical aggregation. Trends Biotechnol. 2013;31(8):448–56.
Mensink MA, Nethercott MJ, Hinrichs WLJ. Influence of miscibility of protein-sugar Lyophilizates on their storage stability. AAPS J. 2016;18(5):1225–32.
Platts L, Falconer RJ. Controlling protein stability: mechanisms revealed using formulations of arginine, glycine and guanidinium HCl with three globular proteins. Int J Pharm. 2015;486(1–2):131–5.
Lim JY, Kim NA, Lim DG, Eun CY, Choi D, Jeong SH. Biophysical stability of hyFc fusion protein with regards to buffers and various excipients. Int J Biol Macromol. 2016;86:622–9.
Thakkar SV, Joshi SB, Jones ME, Sathish HA, Bishop SM, Volkin DB, Middaugh CR. Excipients differentially influence the conformational stability and Pretransition dynamics of two IgG1 Monoclonal antibodies. J Pharm Sci. 2012;101(9):3062–77.
Abbas SA, Sharma VK, Patapoff TW, Kalonia DS. Characterization of antibody-polyol interactions by static light scattering: implications for physical stability of protein formulations. Int J Pharm. 2013;448(2):382–9.
Timasheff SN. Control of protein stability and reactions by weakly interacting Cosolvents: the simplicity of the complicated. Adv Protein Chem. 1998;51:355–428.
Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63(13):1118–59.
Arakawa T, Timasheff SN. Stabilization of protein structure by sugars. Biochemist. 1982;21(25):6536–44.
Abbas SA, Sharma VK, Patapoff TW, Kalonia DS. Opposite effects of Polyols on antibody aggregation: thermal Versus mechanical stresses. Pharm Res. 2012;29(3):683–94.
Ohtake S, Kita Y, Arakawa T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev. 2011;63(13):1053–73.
Fukuda M, Kameoka D, Torizawa T, Saitoh S, Yasutake M, Imaeda Y, Koga A, Mizutani A. Thermodynamic and fluorescence analyses to determine mechanisms of IgG1 stabilization and destabilization by arginine. Pharm Res. 2014;31(4):992–1001.
Vagenende V, Han AX, Mueller M, Trout BL. Protein-associated Cation clusters in aqueous arginine solutions and their effects on protein stability and size. ACS Chem Biol. 2013;8(2):416–22.
Jing Z, Arya MB, James SN. A hydrophobic surface is essential to inhibit the aggregation of a tau-protein-derived Hexapeptide. J Am Chem Soc. 2013;135(18):6846–52.
Filipe V, Hawe A, Carpenter JF, Jiskoot W. Analytical approaches to assess the degradation of therapeutic proteins. Trends Anal Chem. 2013;49:118–25.
Roberts D, Keeling R, Tracka M. Van der walle CF, Uddin S, Warwickert J, Curtis R. The role of electrostatics in protein-protein interactions of a Monoclonal antibody. Mol Pharm. 2014;11(7):2475–89.
Nishi H, Mathäs R, Fürst R, Winter G. Label-free flow Cytometry analysis of Subvisible aggregates in Liquid IgG1 antibody formulations. J Pharm Sci. 2014;103(1):90–9.
Alsenaidy MA, Jain NK, Kim JH, Middaugh CR, Volkin DB. Protein comparability assessments and potential applicability of high throughput biophysical methods and data visualization tools to compare physical stability profiles. Front Pharmacol. 2014;5:1–19.
Joshi SB, Bhambhani A, Zeng YH, Middaugh CR. An empirical phase diagram-high throughput screening approach to the characterization and formulation of biopharmaceuticals. In: Jameel F, Hershenson S, editors. Formulation and process development strategies for manufacturing biopharmaceuticals. New Jersey: Wiley; 2010. p. 174–201.
Arakawa T, Ejima D, Tsumoto K. Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem. 2007;127(1–2):1–8.
Laurence JS, Middaugh CR. Fundamental structures and behaviors of proteins. In: Wang W, Roberts CJ, editors. Aggregation of therapeutic proteins. New Jersey: Wiley; 2010. p. 9–16.
Wen LL, Chen Y, Liao J, Zheng XX, Yin ZN. Preferential interactions between protein and arginine: effects of arginine on tertiary conformational and colloidal stability of protein solution. Int J Pharm. 2015;478(2):753–61.
Borzova VA, Markossian KA, Muranov KO, Polyansky NB, Kleymenov SY, Kurganov BI. Quantification of anti-aggregation activity of UV-irradiated-crystallin. Int J Biol Macromol. 2015;73:84–91.
Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2007;25(7):1487–99.
Mahler HC, Friess W, Grauschopf U, Kiese S. Protein aggregation: pathways, induction factors and analysis. J Pharm Sci. 2009;98(9):2909–34.
Wang Y, Latypov RF, Lomakin A, Meyer JA, Kerwin BA, Vunnum S, Benedek GB. Quantitative evaluation of colloidal stability of antibody solutions using PEG-induced Liquid−Liquid phase separation. Mol Pharm. 2014;11(5):1391–402.
Saito S, Hasegawa J, Kobayashi N, Uchiyama S, Fukui K. Effects of ionic strength and sugars on the aggregation propensity of Monoclonal antibodies: influence of colloidal and conformational stabilities. Pharm Res. 2013;30(5):1263–80.
Lubich C, Malisauskas M, Prenninger T, Wurz T, Matthiessen P, Turecek PL, Scheiflinger F, Reipert BM. A flow-Cytometry-based approach to facilitate quantification, size estimation and characterization of sub-visible particles in protein solutions. Pharm Res. 2015;32(9):2863–76.
Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–36.
Shukla D, Schneider CP, Trout BL. Molecular level insight into intra-solvent interaction effects on protein stability and aggregation. Adv Drug Deliv Rev. 2011;63(13):1074–85.
Charman SA, Mason KL, Charman WN. Techniques for assessing the effects of pharmaceutical excipients on the aggregation of porcine growth hormone. Pharm Res. 1993;10(7):954–62.
Serno T, Carprnter JF, Randolph TW, Winter G. Inhibition of agitation-induced aggregation of a IgG-antibody by Hydroxypropyl-β-Cyclodextrin. J Pharm Sci. 2010;99(3):1193–206.
Eronina TB, Chebotareva NA, Sluchanko NN, Mikhaylova VV, Makeeva VF, Roman SG, Kleymenov SY, Kurganov BI. Dual effect of arginine on aggregation of phosphorylase kinase. Int J Biol Macromol. 2014;68:225–32.
Burstein EA, Vedenkina NS, Ivkova MN. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973;18:263–79.
Haskard CA, Li-Chan ECY. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. J Agric Food Chem. 1998;46(7):2671–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, L., Zheng, X., Wang, X. et al. Bilateral Effects of Excipients on Protein Stability: Preferential Interaction Type of Excipient and Surface Aromatic Hydrophobicity of Protein. Pharm Res 34, 1378–1390 (2017). https://doi.org/10.1007/s11095-017-2152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2152-0