Abstract
Purpose
Measurement of skin absorption of ions requires specific experimental protocols regarding the use of pig skin as a model, the viability of excised skin in water medium over 24 h, the presence of endogenous ions, and evaluation of the contributions of facilitated transport through ion channels and ion transporters.
Method
Absorption experiments of halide anions F−, Cl−, Br− and I− in excised skin were performed in Franz diffusion cells. Experiments were performed on human and porcine skin under various conditions so as to define and validate experimental protocols.
Results
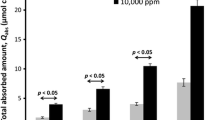
The distributions of endogenous ions and the absorption kinetics of halide ions were similar in both porcine and human skin models. Fresh skin kept its viability over 24 h in salt-free water, allowing experiments following OECD guidelines. Permeation increased in the order F− < Cl− < Br− < I− for all receptor media and skin samples. Absorption was larger in fresh skin due to the transport through chloride channels or exchangers.
Conclusion
Skin absorption experiments of ions in Franz cells rely on working with fresh excised skin (human or porcine) and pure water as receptor fluid. Experiments with chloride blockers or frozen/thawed skin allow discriminating passive diffusion and facilitated transport.







Similar content being viewed by others
Abbreviations
- CaCC:
-
Ca2+ activated Cl− channels
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- ClC:
-
Chloride channel
- D:
-
Dermis
- DF:
-
Donor fluid
- DIDS:
-
4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid
- HBSS:
-
Hank’s balanced salt solution. It contains 1 g.L−1 of glucose in its composition.
- IAA-94:
-
Indanyloxyacetic acid
- IC:
-
Ion chromatography
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- LDH:
-
Lactate deshydrogenase
- NCC:
-
Sodium-chloride symporter (also known as Na+-Cl− co-transporter)
- NIS:
-
Sodium-iodide symporter
- NKCC:
-
Sodium-potassium-chloride co-transporter
- RF:
-
Receptor fluid
- SC:
-
Stratum corneum
- SSG:
-
Serum saline (physiological serum saline solution NaCl 9 g.L−1) containing 1 g.L−1 of glucose
- VE:
-
Viable epidermis
- WG:
-
Ultrapure water (resistivity >18 MΩ.cm at 25°C) containing 1 g.L−1 of glucose
References
Hostynek J-J. Factors determining percutaneous metal absorption. Food Chem Toxicol. 2003;41:327–45.
La Count TD, Kasting GB. Human skin is permselective for the small, monovalent cations sodium and potassium but not for nickel and chromium. J Pharm Sci. 2013;102:2241–53.
Tymen H, Gerasimo P, Hoffschir D. Contamination and decontamination of rat and human skin with plutonium and uranium, studied with a Franz’s chamber. Int J Radiat Biol. 2000;76:1417–24.
Bolzinger MA, Bolot C, Galy G, Chabanel A, Pelletier J, Briançon S. Skin contamination by radiopharmaceuticals and decontamination strategies. Int J Pharm. 2010;402:44–9.
Phipps JB, Pasmanabhan RV, Lattin GA. Iontophoretic delivery of model inorganic and drug ions. J Pharm Sci. 1989;78:365–9.
Denda M, Hosoi J, Asida Y. Visual imaging of ion distribution in human epidermis. Biochem Biophys Res Commun. 2000;272:134–7.
Lin P, Gruenstein E. Pathways of Cl− transport in human fibroblasts. Am J Physiol. 1988;255:112–22.
Mastrocola T, De Luca M, Rugolo M. Characterization of chloride transport pathways in cultured human keratinocytes. Biochim Biophys Acta. 1991;1097:275–82.
Bear CE. Phosphorylation-activated chloride channels in human skin fibroblasts. FEBS Lett. 1988;237:145–9.
Plog S, Mundhenk L, Langbein L, Gruber AD. Synthesis of porcine pCLCA2 protein during late differentiation of keratinocytes of epidermis and hair follicle inner root sheath. Cell Tissue Res. 2012;350:445–53.
Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–68.
Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–47.
Denda M, Inoue K, Inomata S, Denda S. γ-Aminobutyric acid (A) receptor agonists accelerate cutaneous barrier recovery and prevent epidermal hyperplasia induced by barrier disruption. J Investig Dermatol. 2002;119:1041–7.
Denda M, Fuziwara S, Inoue K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J Investig Dermatol. 2003;121:362–7.
Perron B, Rodriguez AM, Leblanc G, Pourcher T. Cloning of the mouse sodium iodide symporter and its expression in the mammary gland and other tissues. J Endocrinol. 2001;170:185–96.
Chen T, Singleton L, Imbert I, Perrin A, Gondran C, McMullen R, et al. Effect of UVB irradiation on intracellular sodium level and chloride channel CFTR expression in normal human keratinocytes and skin biopsies. J Investig Dermatol. 2009;129(supplement 1s):S133,796.
von Zglinicki T, Lindberg M, Roomans GM, Forslind B. Water and ion distribution profiles in human skin. Acta Derm Venereol. 1993;73:340–3.
Forslind B, Lindberg M, Roomans GM, Pallon J, Werner-Linde Y. Aspects on the physiology of human skin: studies using particle probe analysis. Microsc Res Tech. 1997;38:373–86.
Bronaugh RL, Stewart RF, Congdon ER. Methods for in vitro percutaneous absorption studies. II. Animal models for human skin. Toxicol Appl Pharmacol. 1982;62:481–8.
Harrison SM, Barry BW, Dugard PH. Effects of freezing on human skin permeability. J Pharm Pharmacol. 1984;36:261–2.
Bronaugh RL, Stewart RF, Simon M. Methods for in vitro percutaneous absorption studies VII: use of excised human skin. J Pharm Sci. 1986;75:1094–7.
Smith CK, Moore CA, Elahi EN, Smart ATS, Hotchkiss SAM. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol Appl Pharmacol. 2000;168:189–99.
OECD. Guidance document for the conduct of skin absorption studies. OECD series on testing and assessment: number 28. 2004.
Messager S, Hann AC, Goddard PA, Dettmar PW, Maillard JY. Assessment of skin viability: is it necessary to use different methodologies? Skin Res Technol. 2003;9:321–30.
Collier SW, Sheikh NM, Sakr A, Lichtin JL, Stewart RF, Bronaugh RL. Maintenance of skin viability during in vitro percutaneous absorption/metabolism studies. Toxicol Appl Pharmacol. 1989;99:522–33.
Förster M, Bolzinger MA, Rovère MR, Damour O, Montagnac G, Briançon S. Confocal Raman microspectroscopy for evaluating the stratum corneum removal by 3 standard methods. Skin Pharmacol Physiol. 2011;24:103–12.
Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Investig Dermatol. 2004;122:14–9.
EN ISO 10304-1:2009: water quality. Determination of dissolved anions by liquid chromatography of ions. Determination of bromide, chloride, fluoride, nitrate, nitrite, phosphate and sulfate.
Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations-an overview. Skin Pharmacol Appl Skin Physiol. 2000;13:229–34.
Verissimo A, Alves LC, Filipe P, Silva JN, Silva R, Ynsa MD, et al. Nuclear microscopy: a tool for imaging elemental distribution and percutaneous absorption in vivo. Microsc Res Tech. 2007;70:302–9.
Gontier E, Barberet P, Barbotteau Y, Habchi C, Incerti S, Moretto P, et al. Micro-PIXE characterization of different skin models. X-Ray Spectrom. 2005;34:381–8.
Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–8.
Denda M, Tsutsumi M, Inoue K, Crumrine D, Feingold KR, Elias PM. Potassium channel openers accelerate epidermal barrier recovery. Br J Dermatol. 2007;157:888–93.
Denda M, Katagiri C, Hirao T, Maruyama N, Takahashi M. Some magnesium salts and some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch Dermatol Res. 1999;291:560–3.
Halprin KM, Ohkawara A. Lactate production and lactate dehydrogenase in the human epidermis. J Investig Dermatol. 1966;47:222–6.
Reuss L, Hirst BH. Water transport controversies – an overview. J Physiol. 2002;54:1–2.
Lyklema J. Fundamentals of interface and colloid science. Vol 1, Chap 5. London: Academic Press. pp. 5.15–5.31.
Pitzer KS. Electrolyte theory - improvements since Debye and Hückel. Acc Chem Res. 1977;10:371–7.
Guggenheim EA, Turgeon JC. Specific interactions of ions. Trans Faraday Soc. 1955;51:747–61.
Kasting GB, Bowman LA. Electrical analysis of fresh, excised human skin: a comparison with frozen skin. Pharm Res. 1990;7:1141–6.
Burnette RR, Ongpipattanakul B. Characterization of the permselective properties of excised human skin during iontophoresis. J Pharm Sci. 1987;76:765–73.
Wulff H. New light on the “old” chloride channel blocker DIDS. ACS Chem Biol. 2008;3:399–401.
Elisei R, Vivaldi A, Ciampi R, Faviana P, Basolo F, Santini F, et al. Treatment with drugs able to reduce iodine efflux significantly increases the intracellular retention time in thyroid cancer cells stably transfected with sodium iodide symporter complementary deoxyribonucleic acid. J Clin Endocrinol Metab. 2006;91:2389–95.
Weber-Schurholz S, Erhard Wischmeyer E, Laurien M, Jockusch H, Schurholz T, Landry DW, et al. Indanyloxyacetic acid-sensitive chloride channels from outer membranes of skeletal muscle. J Biol Chem. 1993;268:547–51.
ACKNOWLEDGMENTS AND DISCLOSURES
The support of the Ministère de l’Enseignement Supérieur et de la Recherche (France) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paweloszek, R., Briançon, S., Chevalier, Y. et al. Skin Absorption of Anions: Part One. Methodology for In Vitro Cutaneous Absorption Measurements. Pharm Res 33, 1564–1575 (2016). https://doi.org/10.1007/s11095-016-1909-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1909-1