ABSTRACT
Purpose
We previously reported the safety and efficacy in animal experiments of transcutaneous immunization (TCI) using a self-dissolving microneedle patch (MicroHyala; MH) made of hyaluronic acid and collagen. However, this MH was an unsuitable TCI device for the human skin, as collagen is suspected to induce inflammation. In this study, we developed an improved collagen-free MH (new-MH) and conducted clinical study to evaluate the fundamental properties and safety in human.
Methods
Microneedle dissolution, skin irritation, and antigen-specific antibody production about new-MH were measured in mice and/or rats. On the basis of the results, the clinical study was conducted in healthy volunteers to evaluate local and systemic adverse events caused by new-MH application.
Results
We confirmed that the microneedles of new-MH, as well as those on our old-MH that contained collagen, could easily pierce stratum corneum without severe skin irritation, and that TCI using new-MH efficiently increased antibody titer with comparable to TCI using old-MH. Application of new-MH caused no severe adverse reactions in 20 healthy volunteers enrolled in a clinical study.
Conclusions
These results verified that new-MH is a safe TCI device in human, and greatly encouraged us to advance PI/PII clinical studies of antigen-loaded new-MH.
Similar content being viewed by others
INTRODUCTION
Vaccination, which is the only fundamental prophylaxis against illness and death from infectious disease, has greatly contributed to improving human health globally. However, injection as major vaccination system is painful, requires medical personnel with technical skill, and comes with the risk of needle-related diseases and injuries. Moreover, antigen solutions require cold chain storage and transportation systems (1,2). These disadvantages of conventional injections hamper the delivery of vaccination technologies to developing countries. As a result, 4,000 children die every day of diseases that are preventable by vaccination (3). The earlier practice of easy-to-use vaccination method is expected to replace injected vaccinations. In addition, the development of new vaccination systems to enable worldwide mass-treatment is critical to evade pandemics of emerging infectious diseases such as severe acute respiratory syndrome (4), H5N1 highly pathogenic avian influenza (5), and reemergence of infectious diseases such as tuberculosis (6), and malaria (7). One of the innovative methods that resolves these issues and is attracting great attention is transcutaneous immunization (TCI) systems (8,9).
Administration of antigen solution to the skin surface fails to penetrate the stratum corneum and deliver sufficient antigen into the skin (10–12). Therefore, TCI systems must deliver antigen to the epidermal layer and dermis, which contain antigen-presenting cells that induce immune responses. Among many TCI devices, microneedle technologies, which utilize needles of micron-size, are under active research as a novel device that induce immune responses (13). Microneedles can physically penetrate the stratum corneum and directly deliver the antigen into the skin with easy application. Moreover, their application is painless because microneedles do not penetrate the lower dermis, where pain-sensing neurons are densely present. Many research groups have reported the efficacy of TCI using microneedle arrays. However, most conventional microneedles suffer from the risk of fracture, which leaves metal, stainless steel, or silicon microneedle fragments in the skin. (14–16). To address this, novel microneedles that are made of dissolvable or biodegradable materials have been developed by a few research groups including us (17–21). These self-dissolving microneedles leave no bio-hazardous sharp medical waste, and remove the risk of secondary infection by used-needles. Thus, the self-dissolving microneedle array is extremely promising as a novel improved TCI device.
We have developed a self-dissolving microneedle patch (MicroHyala; MH) made of biocompatible hyaluronic acid and collagen (20,21). In a previous study, we reported that TCI using MH effectively induced immune responses against various antigens in animal models. In addition, the application of MH resulted in very little skin irritation in rats. Unfortunately, however, MH is unsuitable for the human skin because the collagen content of MH (old-MH) is suspected to induce inflammation. The major purpose of this study was to realize the practical use of our TCI system. Therefore, we developed a new MH that can be applied to human skin; this new MH does not contain collagen (new-MH). Subsequently, we evaluated its safety and efficacy as a TCI device in animal models and then conducted clinical studies to examine the safety of new-MH on the human skin. Here we showed that our new-MH is a practical and safe device for human immunization.
MATERIALS AND METHODS
Animals
BALB/c mice (female, 6-week-old), Wistar ST rats (female, 6-week-old), Hartley guinea pigs (female, 8-week-old), and HWY hairless rats (female, 7-week-old) were purchased from SLC Inc. (Hamamatsu, Japan). Animals were maintained in the experimental animal facility at Osaka University and experiments were conducted in accordance with the guidelines provided by the Animal Care and Use Committee of Osaka University.
Preparation of Self-Dissolving Microneedle Patches
Self-dissolving microneedle patches were created using micro-molding technologies with sodium hyaluronate as the base material. Our MH, which is referred to as old-MH in this study, was made of sodium hyaluronate (JSQI grade, Kikkoman Biochemifa Company, Tokyo, Japan) and hydrolyzed collagen (Nippi, Inc., Tokyo, Japan), with a weight ratio of 7:3. The self-dissolving microneedle patch without collagen, which is referred to as new-MH in this study, was made of sodium hyaluronate (JP grade, Kikoman Biochemifa Company), Dextran 70 (JP grade, Meito sangyo, Nagoya, Aichi), and Povidone (JPE grade, BASF Japan, Tokyo, Japan) with a weight ratio of 11:8:1. Briefly, solutions of these MH materials were cast into micro-molds and dried in a desiccator at room temperature. Self-dissolving microneedle patches were then separated from the molds. Microneedle lengths were 200 μm (MH200), 300 μm (MH300), 500 μm (MH500), or 800 μm (MH800). These microneedles were photographed using a stereoscopic microscope (VHX-1000 or VHX-D500/510, KEYENCE, Osaka, Japan; Fig. 1a). To form the transcutaneous microneedle patch system, patches with an area of 0.8 cm2 (containing 200 microneedles) were fixed onto 2.3 cm2 adhesive films.
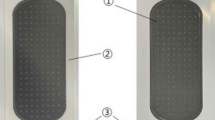
Dimension of new-MHs (a) and procedure of new-MH application to the human skin (b). (a) The new-MHs contain 200 microneedles in an area of 0.8 cm2. New-MH200 and new-MH300 have 200 μm and 300 μm cone-type microneedles of 50 μm and 100 μm lengths, respectively. New-MH500 and new-MH800 contain cone-type microneedles of 500 μm and 800 μm length, respectively. Images of microneedles were taken using a stereoscopic microscope. (b) New-MH was fixed to the plastic case as a new-MH formulation (1), was adhered to the center of a 2.3 cm2 adhesive film (2), was put on skin of the lateral upper arm (3), was applied by impact of a handheld spring-type applicator (4), and was removed 6 h after application (5).
Analysis of Microneedle Dissolution Kinetics
New-MH200, new-MH300, and new-MH800 microneedle patches were applied to the back skin of BALB/c mice and Wistar ST rats for 5, 15, 30, or 60 min as previously described (20). After removing the new-MH patches, microneedles were immediately observed under stereoscopic microscope (VHX-1000, KEYENCE).
Assessment of Skin Irritation
New-MH200, new-MH300, and new-MH800 patches were applied to the back skin of Wistar ST rats for 30 min. Skin was observed and scored for signs of erythema or edema according to the Draize dermal scoring criteria 5 min, and 2, 6, 24, and 48 h after treatment with each new-MH (22,23).
Measurement of Skin Surface Impedance
New-MH200, new-MH300, and new-MH800 patches were applied to the back skin of Wistar ST rats for 30 min. Subsequently, skin surface impedance between application and non-application areas, which indicates the degree of skin barrier dysfunction, was measured using a Pocket Tester (CDM-03D; Custom Inc., Kanagawa, Japan) at 5, 15, 30, 60, and 120 min after removal of new-MH patches.
Vaccine Protocol Using New-MH
Tetanus toxoid (TT) and diphtheria toxoid (DT) were kindly provided by The Research Foundation for Microbial Diseases of Osaka University (Suita, Japan). New-MH800 or old-MH800 with 20 μg TT and 10 μg DT/needles were applied to the back skin of Wistar ST rats for 1 h or 6 h, five times at 2-week intervals. Subcutaneous immunization (SCI) was conducted with the combination of 20 μg TT and 10 μg DT five times at 2-week intervals. Toxoid-specific IgG titers in sera were determined by enzyme-linked immunosorbent assay (ELISA).
New-MH200, new-MH300, and new-MH800 with 1 μg ovalbumin (OVA; Sigma-Aldrich Inc., St. Louis, MO)/needles were applied to the back skin of guinea pigs for 6 h four times at 2-week intervals. The SCI group received 1 μg OVA five times at 2-week intervals and the intraperitoneal immunization (IPI) group was injected with 1 μg OVA containing 5 mg alum (Wako Pure Chemical Industries, Ltd., Osaka, Japan) two times at 2-week intervals. Sera were collected from immunized guinea pigs 2 weeks after final vaccination, and OVA-specific IgG and IgE titers were measured.
Antibody Titer Measurement
Antigen-specific IgG titers were determined by ELISA as previously described (24). Anti-TT and anti-DT IgGs in rats or anti-OVA IgG in guinea pigs was detected by a peroxidase-labeled goat anti-rat IgG antibody or a peroxidase-labeled goat anti-guinea pig IgG antibody (Southern Biotechnology, Birmingham, AL, USA), respectively. End-point titers of antigen-specific antibodies were expressed as the reciprocal log2 of the last dilution that had an absorbance of 0.1 after subtracting the background. Anti-OVA IgE was detected using the following method: Briefly, 96-well titer plates were coated with purified anti-guinea pig IgE monoclonal antibody and were blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich Inc.) in phosphate-buffered saline (PBS). After blocking, serum samples were diluted 16-fold with 1% BSA/PBS and were incubated for 3 h. Plates were washed 3 times with Tris-HCl buffer saline containing 0.1% Tween-20 (TBS-T), and biotinylated OVA diluted in 1% BSA/PBS was added to plates and incubated. After 3 h, plates were washed 3 times with TBS-T and horseradish peroxidase-conjugated goat anti-biotin IgG polyclonal antibody was added. After 3 h, the reaction was developed using a tetramethylbenzidine solution, and color development was terminated by adding 2 N H2SO4. Optical densities were measured at 450−650 nm.
Monitoring of Tetanus Toxin Challenge
Four weeks after the final vaccination, 1-μg tetanus toxin (Sigma-Aldrich Inc.) was injected subcutaneously into the right thigh of TT-immunized rats. The rats were then monitored daily for up to 4 days, and were euthanized in cases of severe paralysis.
Passive Cutaneous Anaphylaxis (PCA) Reaction Against Antisera of Guinea Pigs
The PCA reaction was performed by modifying the method of Hattori et al. (25). Hairless rats were inoculated intradermally with 100 μL of the collected antibody at 1/1, 1/16, 1/64, or 1/128 dilutions. After 24 h, PCA was elicited by intravenous injection of a 1-mL solution containing 2.5 mg OVA and 5 mg of Evans blue. After 0.5 h, the diameter of the skin reaction spots, which occurred following extravasation of dye at the site of serum injection, were measured using the following formula: diameter of spot = (long diameter + short diameter)/2.
Active Systemic Anaphylaxis (ASA) of Immunized Guinea Pigs
Measurement of ASA was performed using the modified method of Aida et al. (26).Guinea pigs were inoculated intravenously with OVA (2 mg/kg) 2 weeks after the final vaccination. Anaphylactic symptoms and the ratio of survival were scored after 0.5 and 24 h, respectively, in guinea pigs using the ASA scoring system: 0, symptomless; 1, rubbing face and ears, cough more than 2 times; 2, cyanosis caused on eye or ear, intense spasm and tumbling; 3, death.
Clinical Study Design for Safety Assessment of New-MH
Twenty healthy male volunteers (25–56 years of age) were enrolled in the study. Written informed consent was received from all volunteers before enrollment. Four types of self-dissolving microneedle patches (new-MH300, new-MH500, new-MH800, and new-MH-needleless) were applied to the skin of left lateral upper arms of 20 subjects for 6 h using a handheld applicator at 12.8 N/200 microneedles (Fig. 1b). To assess local adverse effects, skin irritation reactions were scored 2, 3, 7, and 30 days after applying the patch, according to the classification of the International Contact Dermatitis Research Group (ICDRG) (27,28):−, negative reaction; ?+, doubtful reaction, faint erythema only; +, weak (non-vesicular) positive reaction, erythema, infiltration and possibly papules; ++, strong (vesicular) positive reaction, erythema, infiltration, papules, vesicles; +++, extreme positive reaction, bullous reaction; IR, irritant reaction. In 17 volunteers, the presence of purpura was observed by disappearing erythema after pressure was applied to the skin with a glass plate. Blood samples were collected at day 0 and day 2. General peripheral blood tests and biochemical tests of liver and renal function were performed to evaluate the presence of systemic adverse reactions. All clinical procedures were approved by Institutional Review Board for Clinical Research, Osaka University Graduate School of Medicine.
Observation of New-MH Application Sites by In Vivo Confocal Laser-Scanning Microscopy
New-MH500 and new-MH800 patches were removed immediately, 1 h, or 2 h after application. Application sites were observed using a confocal laser-scanning microscope (Vivascope 1500; Lucid Inc., Rochester, NY, USA) immediately or 1 h after patch removal. Photographs of 0.5 μm × 0.5 μm areas were taken for every 1 μm depth, and a z-axis image was constructed at the depth of 100 μm. In addition, a wide range of 4 × 4 mm images were obtained; numbering 64 photographs from 8 vertical × 8 horizontal pieces.
Analysis of Microneedle Dissolution Kinetics in Humans
New-MH800 patches were applied to the skin of the left lateral upper arms of three volunteers, and were removed 1 h and 6 h after application. Microneedle patches were immediately observed using a stereoscopic microscope (VHX-1000, KEYENCE).
Measurement of Transepidermal Water Loss (TEWL)
New-MH800 patches were applied to the skin of the left lateral upper arms of three volunteers, and were removed immediately, 1 h, and 6 h after application. As controls, new-MH-needleless patches were applied for 6 h. To evaluate the degree of skin barrier dysfunction, TEWL was measured in the sites of application using a Mobile Tewameter (MSC100/TM300; Courage +Khazaka electronic GmbH, Köln, Germany).
Assessment of Pain
The pain associated with application of microneedle patches was expressed numerically using a Visual Analogue Scale (VAS) from 0 (no pain) to 100 (unbearable pain).
RESULTS
Comparison of Fundamental Characteristics of New-MH and Old-MH
We investigated dissolution kinetics of microneedles in mice and rats, whose skins differ in hardness and in thickness by approximately 10 μm. New-MH200 and new-MH300 microneedle tips dissolved rapidly and microneedles on new-MH800 resulted in a 50% reduction in length after 5 min in both mice and rats (Fig. 2a and b). The dissolution of microneedles progressed with application time, and all new-MH had dissolved completely after 60 min. These dissolution kinetics and technique for the insertion of microneedles were identical to those of old-MHs independent of the components. We evaluated erythema and edema at the application site using the Draize scoring system 30 min after applying new-MHs. Five minutes after patch removal, slight erythema was observed in 1 of 3 rats treated with new-MH200, in 2 of 3 rats treated with new-MH300, and in all rats treated with new-MH800. These observations suggest that erythema is exacerbated by needle length (Fig. 2c). However, erythema disappeared within 48 h, and edema was not observed in any rats. Thus, application of new-MH caused only minor irritation of the skin. Using skin impedance measurements, we observed the recovery of skin with microneedle puncture holes. Application of either new-MH200 or new-MH300 decreased skin impedance to 60–80%, whereas application of new-MH800 or tape-strips decreased skin impedance to 20% (Fig. 2d). The impedance of tape-stripped skin did not recover within 120 min. On the other hand, skin impedance recovered within 60 min after removal of new-MH200 or new-MH300 patches, and within 120 min after removal of new-MH800 patches. Therefore, puncture holes that were caused by insertion of microneedles closed within a few hours of new-MH application, indicating a low risk of secondary infection. Given similar fundamental characteristics of new-MH and old-MH, we conclude that new-MH is a safe and minimally invasive device.
Needle-dissolution kinetics of new-MH, and skin status after application of new-MH. (a and b) New-MH200, new-MH300, and new-MH800 were applied on the back skin of BALB/c mice (a) or Wistar ST rats (b) for the indicated times. After removal of new-MH, the microneedles remaining on each new-MH were photographed using a stereoscopic microscope. (c) New-MH200, new-MH300, or new-MH800 were applied to the back skin of Wistar ST rats for 30 min. The degree of erythema on the skin of Wistar ST rats was scored using the Draize scoring system: 0, no erythema or edema; 1, very slight erythema and/or barely perceptible edema; 2, well-defined erythema and/or slight edema; 3, moderate to severe erythema or moderate edema, and 4, severe erythema and/or edema, 5 min, 2 h, 6 h, 24 h, or 48 h after removing new-MHs. The mean score is shown as a bar. Each panel shows photographs of application areas 5 min after new-MH removal. (d) Skin impedance of new-MH application areas and non-application areas was measured 5, 15, 30, 60, and 120 min after 30-min applications. As controls, back skin of Wistar ST rats were tape-stripped. Data are expressed as mean ± SE of data from three rats.
Immune Responses Induced by TCI Using Antigen-Loaded New-MH
We selected MH800, and compared vaccine delivery efficiency between TCI using new-MH and old-MH over 1 h or 6 h. The profile of anti-toxoid IgG antibody production following application of new-MH was similar to that of old-MH (Fig. 3a). Thus, we confirmed that the components of microneedles did not influence the intended immune response. In addition, there was no significant difference between the 1-h and 6-h application periods, indicating that the complete dissolution of microneedles within 1 h of application was sufficient to deliver antigen into the skin and to achieve effective vaccination. To further assess vaccination efficacy, we evaluated whether toxoid-specific antibodies, produced by application of new-MH, neutralized tetanus toxin. Immunized rats were resistant to the lethal toxin after tetanus toxin challenge, whereas the rats treated with antigen-free new-MH800 died (Table I). Therefore, we have confirmed that antigen-contained new-MH patches induce a protective immune response against infectious diseases as effectively as those of old-MH (21).
Immune responses induced by antigen loaded new-MH formulations. (a) The new-MH800 (♢ and ♦) or old-MH800 (□ and ■) with 20 μg TT and 10 μg DT/needles were applied to the back skin of Wistar ST rats five times at 2-week intervals for 1 h (♢ and □) or 6 h (♦ and ■). Wistar ST rats were subcutaneously injected with equivalent TT and DT doses (△). At the indicated points, sera collected from these rats were assayed for TT or DT IgG titers by ELISA. Data are expressed as mean ± SE of results from five rats. Arrows indicate the vaccination point. (b) Hartley guinea pigs were treated with new-MH200, new-MH300, or new-MH800 containing 1 μg OVA/needles for 6 h four times at 2-week intervals, or were subcutaneously immunized with 1 μg OVA (SCI) five times at 2-week intervals. As positive controls, Hartley guinea pigs were immunized by intraperitoneal injection of 1-μg OVA and 5-mg Alum (IPI) twice at 2-week intervals. Two weeks after the final vaccination, sera were collected from these animals and were assayed for OVA-specific IgG titers, and O.D. value of IgE (16-fold dilution) by ELISA. For the PCA reaction, non-sensitized Wistar ST rats were injected with sera from immunized-guinea pigs. Twenty-four hours later, these rats were intravenously injected with Evans blue and OVA, and a leak blue spot at the injection site was measured 30 min later. Data are expressed as mean ± SE of results from 4 to 6 guinea pigs. In ASA assessments, guinea pigs were intravenously injected with OVA a month after the final vaccination, and the performance status of guinea pigs was scored using the ASA scoring system. N.D.; not detected.
To assess unintended immune responses, we evaluated antigen-specific IgE production in guinea pigs, which is mainly used to identify allergic reactions. Anti-OVA IgG titer increased in all immunized guinea pigs, and the TCI and SCI groups showed little induction of anti-OVA IgE (Fig. 3b). In addition, we conducted PCA and ASA analyses to assess allergic reactions. In the PCA analysis, antigen-specific Evans blue leak from the injection site was measured in sera from immunized guinea pigs. In the IPI group, a positive PCA reaction (>5 mm) was observed; the diameter of the ceruleus was 6.9 mm in a 1/128 dilution of sera. On the other hand, sera of TCI and SCI groups did not give positive PCA reactions, even in undiluted sera. In ASA assessments, allergic reactions, which appeared after injection of antigen into immunized guinea pigs, was evaluated. Positive control guinea pigs died immediately, but guinea pigs of the TCI and SCI groups remained alive. The ASA scores of TCI and SCI groups were all below 1, although minor anaphylactic symptoms were observed in TCI-treated animals following application of new-MH800, as well as in SCI animals. Because these results showed that TCI using new-MH induced antigen-specific immune responses and did not cause allergic reactions, we conclude that new-MH is a safe and efficacious device for practical use in TCI system.
State of the Human Skin After Application of New-MHs
In animal experiments, we showed that the new-MH device is practical and suitable for TCI. Subsequently, we conducted a clinical study to demonstrate the application of new-MHs to human skin. We examined skin using a confocal laser-scanning microscope immediately after 5-s application of new-MH500 or new-MH800 to the human skin. Puncture holes were distinct on the skin surface, and the delivery depth was at least 100 μm (Fig. 4a). As the thickness of human stratum corneum is approximately 10–20 μm, this indicates that the microneedles of new-MH were able to deliver antigen into the epidermal layer of the human skin. Given that the thickness and water content of skin differs between animals and humans, we confirmed the solubility of microneedles in humans at various times after application. After 1 h, the microneedles had completely dissolved in 2 of 3 subjects, and their length was reduced by 50% in the other subject (Fig. 4b). In all subjects, the microneedles fully dissolved within 6 h after new-MH application, although complete dissolution times varied between individuals. These data showed that the new-MH patches deliver antigen to humans in 6 h. Next, we monitored the closing of puncture holes by measuring TEWL as an index of skin barrier dysfunction. TEWL of treated skin increased immediately after new-MH800 patches were removed and decreased gradually with time (Fig. 4c), indicating rapid recovery of skin barrier function. After 6-h application of new-MH patches, TEWL was lower than that after 5-s or 1-h application. In addition, confocal laser-scanning microscopy showed that the puncture holes tended to close with longer new-MH application periods (Supplementary Material Fig. S1). Hence, given the dissolution kinetics, we did not consider barrier dysfunction or the risk of secondary infection after 6 h applications of new-MH.
Status of skin treated with new-MHs, and needle-dissolution of new-MH in humans. (a) New-MH800 or new-MH500 were applied to the skin of the left lateral upper arms of two healthy volunteers for 5 s, and skin images were immediately photographed using in vivo confocal scanning laser microscopy. (b) New-MH800 was applied to the skin of the left lateral upper arms of three healthy volunteers for 1 h or 6 h, and microneedle patches were immediately observed using a stereoscopic microscope. (c) New-MH800 was applied to the skin of the left lateral upper arms of three healthy volunteers for 5 s (□), 1 h (△), or 6 h (○). As a control, new-MH-needleless was applied for 6 h (●). At the indicated time after new-MH removal, TEWL of the application sites was measured. Data are expressed as mean ± SE of results from three subjects. ♦; TEWL of untreated skin. (d) New-MH-needleless, new-MH300, new-MH500, and new-MH800 were applied to the skin of left lateral upper arms of 17 healthy volunteers. Subjects were asked to grade the pain experienced using a VAS from 0 (no pain) to 100 (unbearable pain). Data are expressed as mean ± SE of results from 17 subjects.
To further assess the safety and utility of new-MH patches, we evaluated the pain of new-MH application using VAS assessments in 17 subjects. Among subjects treated with new-MH-needleless, new-MH300, new-MH500, and new-MH800, no significant differences in VAS scores were identified by the Steel–Dwass test (Fig. 4d). We assumed that the VAS scores associated with new-MH-needleless, which did not have microneedles, resulted from the impact of the handheld spring-type applicator. The VAS scores for each new-MH were low, suggesting that the insertion of microneedles into the skin was minimally painful.
Finally, we assessed local and systemic adverse effects of new-MH application for 6 h in humans. Two days after each new-MH application, faint erythema was observed in 1 subject treated with new-MH300, 12 subjects treated with new-MH500, and 13 subjects treated with new-MH800 (Table II). However, these local responses disappeared in most subjects within 7 days, and skin condition recovered in all subjects within 30 days after application. After application of new-MH500 and new-MH800, purpura, which is caused by capillary damage, was observed in about a half of the subjects. These symptoms disappeared within 30 days, except in the case of one subject with remaining pigmentation that was clinically unproblematic. Application of new-MHs did not cause any systemic adverse effects as determined by a general peripheral blood test and biochemical tests of liver and renal function (Supplementary Material Fig. S2). Thus, we have demonstrated that TCI using new-MH can be applied to humans without severe local or systemic adverse responses.
DISCUSSION
In this study, we prepared new-MH without collagen and investigated the safety of new-MH application to the human skin. We previously reported that old-MH, which contained collagen, is a safe and efficacious device in animal experiments (20,21). However, collagen is suspected to cause allergies in humans. The microneedles of new-MH were inserted into animal skin, and dissolved completely within 1 h as did old-MH. Severe local responses were not observed after application of new-MH. Skin impedance decreased immediately after new-MH application and recovered within 120 min, indicating that the puncture holes caused by new-MH application close rapidly. In addition, we verified that new-MH containing TT and DT induced immune responses that were equal to those produced by old-MH and subcutaneous immunization. Thus, we demonstrated safe application of new-MH to animal skin, effective delivery of the antigen into the skin, and induction of antigen-specific antibodies.
Some researchers have indicated that TCI is more effective than conventional subcutaneous or intramuscular injections (29,30), and in particular, strongly induces Th2 responses (31,32). Because the production of IgE antibody was apprehended during induction of the immune response, we conducted an allergy test of new-MH using guinea pigs. Application of new-MHs did not significantly increase IgE antibodies, and induced an antigen-specific anaphylactic reaction, indicating that new-MH did not induce allergic responses. Thus, we hypothesized that new-MH could be applied to the human skin safely.
Based on these results, we conducted a clinical study of new-MH on the human skin. The microneedles on new-MH successfully penetrated the human skin, which differs in thickness and water content to animal skin, and dissolved completely within 6 h. In animal experiments, we confirmed that there were no significant differences in immune responses between 1-h and 6-h applications of new-MH. Because the application period, in which complete dissolution occurs, may effect induction of the intended immune response, we confirmed that the microneedles of new-MH delivered sufficient antigen into the skin, and induced an immune response in 6 h. Moreover, in TEWL assessments of skin barrier function, skin treated with new-MH for 6 h was more functional than the skin treated for 1 h. Therefore, we decided that 6 h application is necessary to maximize efficacy of antigen delivery and recovery of skin barrier function.
Subsequently, we assessed local and systemic adversities of new-MH application for 6 h in 20 human subjects. Although application of new-MH caused slight erythema in a few subjects, most reactions disappeared within 30 days. In addition, severe systemic adverse events were not observed in blood tests. Thus, we have shown that this new-MH device can be safely applied to the human skin.
In recent years, Intanza/IDflu (Sanofi Pasteur) has been approved as a novel method for influenza vaccination. Intanza/IDflu uses a “Soluvia” (Becton Dickinson) device, which has a single 1.5-mm long needle that allows intradermal injection of vaccine. While Intanza/IDflu has proven skin targeting vaccination efficacy, the use of needles that are longer than 1 mm has the disadvantage of pain. Therefore, the development of a painless vaccination system using microneedles of less than 1-mm length is required. In previous studies, various microneedles such as hollow microneedles and coating microneedles, have been developed (16,33). However, the hollow needle formulation requires cold chain storage and transportation of antigen solutions, and the coating microneedle formulation is limited by the quantity of antigen that can be coated onto microneedle surfaces. To date, these microneedle technologies have not become practical to use. Our new-MH has the potential to overcome these problems, because antigen is contained within the microneedles. Indeed, the present data greatly contribute to the practical use of microneedle devices, and we are performing clinical studies to assess safety and efficacy of new-MH in the delivery of seasonal trivalent influenza HA antigens. Furthermore, applicators for self-administration are being developed for microneedle formulations.
CONCLUSIONS
We prepared collagen-free new-MH for clinical use, and confirmed that there were no differences in safety and efficacy between new-MH and old-MH. In addition, this study shows that new-MH is safely applicable to the human skin. We expect that this innovative new-MH TCI system for vaccine delivery will greatly decrease the mortality and morbidity that is associated with preventable infectious diseases.
Abbreviations
- ASA:
-
active systemic anaphylaxis
- BSA:
-
bovine serum albumin
- DT:
-
diphtheria toxoid
- ELISA:
-
enzyme-linked immunosorbent assay
- ICDRG:
-
international contact dermatitis research group
- IPI:
-
intraperitoneal immunization
- MH:
-
MicroHyala
- OVA:
-
ovalbumin
- PBS:
-
phosphate-buffered saline
- PCA:
-
passive cutaneous anaphylaxis
- SCI:
-
subcutaneous immunization
- TBS-T:
-
Tris-HCl buffered saline containing 0.1% Tween-20
- TCI:
-
transcutaneous immunization
- TEWL:
-
transepidermal water loss
- TT:
-
tetanus toxoid
- VAS:
-
visual analogue scale
REFERENCES
Azad N, Rojanasakul Y. Vaccine delivery-current trends and future. Curr Drug Deliv. 2006;3(2):137–46.
Kersten G, Hirschberg H. Needle-free vaccine delivery. Expert Opin Drug Deliv. 2007;4(5):459–74.
UNICEF’s work on immunisation. http://www.unicef.org.uk/UNICEFs-Work/What-we-do/UNICEFs-work-on-immunisation/
Leppin A, Aro AR. Risk perceptions related to SARS and avian influenza: theoretical foundations of current empirical research. Int J Behav Med. 2009;16(1):7–29.
Haque A, Lucas B, Hober D. Influenza A/H5N1 virus outbreaks and prepardness to avert flu pandemic]. Ann Biol Clin. 2007;65(2):125–33.
Valadas E, Antunes F. Tuberculosis, a re-emergent disease. Eur J Radiol. 2005;55(2):154–7.
Campbell CC. Malaria: an emerging and re-emerging global plague. FEMS Immunol Med Microbiol. 1997;18(4):325–31.
Glenn GM, Scharton-Kersten T, Alving CR. Advances in vaccine delivery: transcutaneous immunisation. Expert Opin Investig Drugs. 1999;8(6):797–805.
Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9(1):99–103.
Rougier A, Rallis M, Krien P, Lotte C. In vivo percutaneous absorption: a key role for stratum corneum/vehicle partitioning. Arch Dermatol Res. 1990;282(8):498–505.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Barry BW. Breaching the skin’s barrier to drugs. Nat Biotechnol. 2004;22(2):165–7.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–5.
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52.
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24.
Sullivan SP, Koutsonanos DG, Del Pilar MM, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–20.
Matsuo K, Yokota Y, Zhai Y, Quan YS, Kamiyama F, Mukai Y, et al. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J Control Release. 2012;161(1):10–7.
Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, Quan YS, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release. 2012;160(3):495–501.
Draize J, Woodard G, Calvery H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–90.
Barile FA. Validating and troubleshooting ocular in vitro toxicology tests. J Pharmacol Toxicol Methods. 2010;61(2):136–45.
Kunisawa J, Takahashi I, Okudaira A, Hiroi T, Katayama K, Ariyama T, et al. Lack of antigen-specific immune responses in anti-IL-7 receptor alpha chain antibody-treated Peyer’s patch-null mice following intestinal immunization with microencapsulated antigen. Eur J Immunol. 2002;32(8):2347–55.
Hattori H, Yamaguchi F, Wagai N, Kato M, Nomura M. An assessment of antigenic potential of beta-lactam antibiotics, low molecular weight drugs, using guinea pig models. Toxicology. 1997;123(1–2):149–60.
Aida T, Ishikawa N, Shinkai K. Differences in immune responses to antibiotics in three guinea-pig strains. J Toxicol Sci. 1997;22(5):439–45.
Fregert S. Manual of contact dermatitis. 2nd ed. Copenhagen: Munksgaard; 1981.
Ivens U, Serup J, O’Goshi K. Allergy patch test reading from photographic images: disagreement on ICDRG grading but agreement on simplified tripartite reading. Skin Res Technol. 2007;13(1):110–3.
Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–301.
Ansaldi F, Durando P, Icardi G. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin Biol Ther. 2011;11(3):415–27.
Tada Y, Asahina A, Fujita H, Sugaya M, Tamaki K. Langerhans cells do not produce interferon-gamma. J Invest Dermatol. 2003;120(5):891–2.
Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17(4):273–83.
Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27(3):454–9.
ACKNOWLEDGMENTS AND DISCLOSURES
We are grateful to The Research Foundation for Microbial Diseases of Osaka University (Suita, Japan) for providing tetanus and diphtheria toxoids. This work was supported by the Advanced research for medical products Mining Program of the National Institute of Biomedical Innovation (NIBIO), by Health and Labour Sciences Research Grants in Research on New Drug Development from the Ministry of Health, Labour and Welfare, and by a Grant-in-Aid for Scientific Research (B) (24390041) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Confocal microscope images of the human skin after application of new-MH. New-MH800 or new-MH500 were applied to the skin of left lateral upper arms of 2 healthy volunteers for 5 s, 1 h, or 2 h. Immediately, or 1 h later, skin photographs were taken using in vivo confocal scanning laser microscopy. Numerical values shown at upper right of each photograph indicate the number of puncture holes (arrow heads) in the field of view. (PPTX 1109 kb) (PDF 509 kb)
Supplemental Fig. 2
Blood tests following application of new-MH. Blood samples were collected before new-MH application (BA) and 48 h after application (48 h), and general peripheral blood tests (MCV; mean corpuscular volume, MCH; mean corpuscular hemoglobin MCHC; mean corpuscular hemoglobin concentration), and biochemical tests of liver and renal function (CRP, C-reactive protein; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; GGT, gamma-glutamyltransferase) were conducted. Solid spot; value of each subject. Solid bar; mean ± SD. Dash bar; standard value. (PDF 44 kb)
Rights and permissions
About this article
Cite this article
Hirobe, S., Azukizawa, H., Matsuo, K. et al. Development and Clinical Study of a Self-Dissolving Microneedle Patch for Transcutaneous Immunization Device. Pharm Res 30, 2664–2674 (2013). https://doi.org/10.1007/s11095-013-1092-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1092-6