Abstract
Purpose
The purpose of this study was to explore the feasibility of linking the pharmacokinetic profile of a drug with its gastrointestinal behavior by simultaneously monitoring plasma and intraluminal drug concentrations. Fosamprenavir, a phosphate ester prodrug of the poorly water-soluble HIV-inhibitor amprenavir, was selected as model compound.
Methods
A single tablet of fosamprenavir (Telzir®) was administered to 5 volunteers in the fasted and fed state (simulated by intake of a nutritional drink). Gastric and duodenal fluids were aspirated in function of time and characterized with respect to the concentration of (fos)amprenavir, inorganic phosphate and pH. In parallel, blood samples were collected and analyzed for amprenavir.
Results
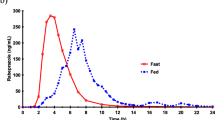
The observed plasma concentration-time profiles suggested a food-induced delay in the absorption of amprenavir: in the fed state, mean t max increased by more than 150 min compared to the fasted state. A similar delay was seen in the duodenal appearance of fosamprenavir (concentrations in mM-range) and, after dephosphorylation, amprenavir (concentrations below 160 μM). This observation could be related to the behavior of fosamprenavir in the stomach. In the fasted state, gastric dissolution of fosamprenavir started immediately, resulting in a C max of 4 ± 2 mM after 43 ± 15 min; however, in the fed state, the fosamprenavir concentration remained below 20 μM for the first 90 min after drug intake. The postponed gastric dissolution may be attributed to a food-induced delay in tablet disintegration.
Conclusion
For the first time, the pharmacokinetic profile of a drug was monitored in parallel with its gastrointestinal concentrations. The observed food effect in the plasma concentration-time profile of amprenavir after intake of its phosphate ester prodrug could be related to a food-induced delay in gastric dissolution of fosamprenavir.







Similar content being viewed by others
References
I. R. Wilding, A. J. Coupe, and S. S. Davis. The role of gamma-scintigraphy in oral drug delivery. Adv. Drug Deliv. Rev. 46:103–124 (2001).
W. Weitschies, O. Kosch, H. Monnikes, and L. Trahms. Magnetic marker monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv. Drug Deliv. Rev. 57:1210–1222 (2005).
L. Bonlokke, L. Hovgaard, H. G. Kristensen, L. Knutson, and H. Lennernas. Direct estimation of the in vivo dissolution of spironolactone, in two particle size ranges, using the single-pass perfusion technique (Loc-I-Gut) in humans. Eur. J. Pharm. Sci. 12:239–250 (2001).
N. Petri, C. Tannergren, B. Holst, F. A. Mellon, Y. Bao, G. W. Plumb, J. Bacon, K. A. O’Leary, P. A. Kroon, L. Knutson, P. Forsell, T. Eriksson, H. Lennernas, and G. Williamson. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab. Dispos. 31:805–813 (2003).
C. Tannergren, T. Knutson, L. Knutson, and H. Lennernas. The effect of ketoconazole on the in vivo intestinal permeability of fexofenadine using a regional perfusion technique. Br. J. Clin. Pharmacol. 55:182–190 (2003).
E. Bergman, P. Forsell, A. Tevell, E. M. Persson, M. Hedeland, U. Bondesson, L. Knutson, and H. Lennernas. Biliary secretion of rosuvastatin and bile acids in humans during the absorption phase. Eur. J. Pharm. Sci. 29:205–214 (2006).
J. Brouwers, F. Ingels, J. Tack, and P. Augustijns. Determination of intraluminal theophylline concentrations after oral intake of an immediate- and a slow-release dosage form. J. Pharm. Pharmacol. 57:987–996 (2005).
J. Brouwers, J. Tack, F. Lammert, and P. Augustijns. Intraluminal drug and formulation behavior and integration in in vitro permeability estimation: a case study with amprenavir. J. Pharm. Sci. 95:372–383 (2006).
E. S. Furfine, C. T. Baker, M. R. Hale, D. J. Reynolds, J. A. Salisbury, A. D. Searle, S. D. Studenberg, D. Todd, R. D. Tung, and A. Spaltenstein. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob. Agents Chemother. 48:791–798 (2004).
C. Falcoz, J. M. Jenkins, C. Bye, T. C. Hardman, K. B. Kenney, S. Studenberg, H. Fuder, and W. T. Prince. Pharmacokinetics of GW433908, a prodrug of amprenavir, in healthy male volunteers. J. Clin. Pharmacol. 42:887–898 (2002).
M. B. Wire, M. J. Shelton, and S. Studenberg. Fosamprenavir : clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin. Pharmacokinet. 45:137–168 (2006).
J. Brouwers, J. Tack, and P. Augustijns. In vitro behavior of a phosphate ester prodrug of amprenavir in human intestinal fluids and in the Caco-2 system: Illustration of intraluminal supersaturation. Int. J. Pharm. (2007, in press). DOI: 10.1016/j.ijpharm.2006.12.011.
S. A. Müller-Lissner, C. J. Fimmel, N. Will, W. Müller-Duysing, F. Heinzel, and A. L. Blum. Effect of gastric and transpyloric tubes on gastric emptying and duodenogastric reflux. Gastroenterology 83:1276–1279 (1982).
W. Weitschies, R. Wedemeyer, O. Kosch, K. Fach, S. Nagel, E. Soderlind, L. Trahms, B. Abrahamsson, and H. Monnikes. Impact of the intragastric location of extended release tablets on food interactions. J. Control. Release 108:375–385 (2005).
J. M. Treluyer, G. Bowers, N. Cazali, M. Sonnier, E. Rey, G. Pons, and T. Cresteil. Oxidative metabolism of amprenavir in the human liver. Effect of the CYP3A maturation. Drug Metab. Dispos. 31:275–281 (2003).
S. L. Ford, M. B. Wire, Y. Lou, K. L. Baker, and D. S. Stein. Effect of antacids and ranitidine on the single-dose pharmacokinetics of fosamprenavir. Antimicrob. Agents Chemother. 49:467–469 (2005).
B. Abrahamsson, T. Albery, A. Eriksson, I. Gustafsson, and M. Sjoberg. Food effects on tablet disintegration. Eur. J. Pharm. Sci. 22:165–172 (2004).
Acknowledgements
This study was supported by grants from: (1) ‘Fonds voor Wetenschappelijk Onderzoek’, Flanders, (2) ‘Onderzoeksfonds’ of the K.U.Leuven, Belgium, (3) Eli Lilly and Company and (4) Organon NV. We also wish to thank Rita Vos (Center for Gastroenterologic Research, University Hospitals Leuven, Belgium) for her assistance during the in vivo studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brouwers, J., Tack, J. & Augustijns, P. Parallel Monitoring of Plasma and Intraluminal Drug Concentrations in Man After Oral Administration of Fosamprenavir in the Fasted and Fed State. Pharm Res 24, 1862–1869 (2007). https://doi.org/10.1007/s11095-007-9307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9307-3