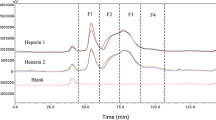
Low-molecular-weight heparin was synthesized by depolymerization of high-molecular-weight heparin with nitrous acid. Preparative chromatography was used to isolate low-molecular-weight heparins with narrow molecular-weight distributions, as confirmed by size-exclusion HPLC. The average molecular weight of the products according to capillary viscometry decreased with synthesis time (4 h) from 14.0 to 3.4 kDa. Fractions with molecular-weight characteristics, ratios of sulfo- to carboxyl groups, and specific activities corresponding to those of low-molecular-weight heparin isolated from commercially available Fragmin® were isolated among the products. Low-molecular-weight heparins with characteristics analogous to those of Dalteparin and Nadroparin substances could be obtained depending on the treatment time of high-molecular- weight heparin with nitrous acid in acidic medium.



Similar content being viewed by others
References
L. E. Frumin, A. V. Panov, S. A. Kedik, et al., Biofarm. Zh., 10(2), 10 – 22 (2018).
A. A. Gadel’shina, Int. J. Appl. Fundam. Res., 9, 354 – 356 (2018).
O. L. Romanova and N. V. Sturov, Trudnyi Patsient, 9(10), 32 – 36 (2011).
S. A. Golubev, Nov. Khir., 15(3), 83 – 90 (2007).
H. E. Conrad, Heparin-Binding Proteins, Academic Press, New York (1998).
J. Hirsch, P. N. Shaklee, et al., US Pat. 5,767,269, Jun. 16, 1998.
RU Pat. No. 133,253, 2013.
L. E. Frumin, A. V. Panov, S. A. Kedik, et al., Khim.-farm. Zh., 53(9), 40 – 45 (2019); Pharm. Chem. J., 53, 852 – 857 (2019).
L. Rodriguez-Manas, R. Gomez-Huelgas, F. Veiga-Fernandez, et al., Clin. Drug Invest., 30(5), 337 – 345 (2010).
J. L. Balibrea, J. Altimiras, and I. Larruzea, Int. J. Surg., 5(2), 114 – 119 (2007).
I. S. Yavelov, Klin. Farmakol. Ter., 19(1), 56 – 63 (2010).
E. N. Kondrashenko, A. V. Butrov, and A. B. But-Gusaim, Trudnyi Patsient, 10(11), 33 – 36 (2012).
L. E. Frumin, G. N. Khlyabich, G. V. Avramenko, et al., Butler. Soobshch., 38(4), 146 – 152 (2014).
A. L. Berkovskii, E. V. Sergeeva, A. V. Suvorov, et al., Determination of Heparin Activity, Methodical Instructions [in Russian], Gematologicheskii Nauchnyi Tsentr, Moscow (2011).
W. E. Barnett, US Pat. 4,351,938, Sept. 28, 1982.
S. E. Lasker and S. S. Stivala, Arch. Biochem. Biophys., 115, 360 – 372 (1966).
Nadroparin Calcium, European Pharmacopoeia 8.0., 3526 – 3529 (2014).
Dalteparin Sodium, European Pharmacopoeia 8.0., 2603 – 2604 (2014).
K. Kato and T. Peters (eds.), NMR in Glycoscience and Glycotechnology, Royal Society of Chemistry (2017).
ACKNOWLEDGMENTS
The work was financially supported by the Ministry of Education and Science of the Russian Federation under the project part of a State Task for 2017 – 2019 (Project 15.2463.2017/4.6).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 3, pp. 43 – 47, March, 2021.
L. E. Frumin is deceased
Rights and permissions
About this article
Cite this article
Yur’eva, K.P., Frumin, L.E., Baeva, E.A. et al. Molecular Weight Characteristics and Anticoagulant Activity of Low-Molecular-Weight Heparin Obtained By Nitrous Acid Depolymerization. Pharm Chem J 55, 295–299 (2021). https://doi.org/10.1007/s11094-021-02414-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02414-z