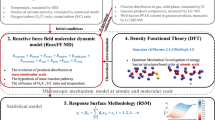
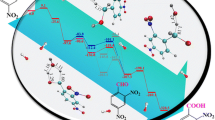
Abstract
This study uses density functional theory (DFT) simulations to predict the main pathways by which hydroxyl (OH) radicals oxidize phenol into monohydroxylated products during an electrical discharge directly in or contacting water. The calculated activation energies and reaction rate constants indicate that phenol ring H abstraction is less likely to occur than OH addition, which will be the fastest in the ortho and para positions. The chain propagation with molecular oxygen of such formed ortho and para radicals will result in the production of hydroquinone and catechol, which are, concurrently, the most likely products of phenol degradation by OH radicals. Electron transfer reactions between dihydroxycyclohexadienyl radicals and plasma oxidative species are another important reaction mechanism which may be contributing significantly to the formation of products. Good agreement between computed kinetic and experimental data demonstrates the feasibility of applying DFT to investigate chemical reaction mechanisms.


Similar content being viewed by others
References
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals ·OH/·O− in aqueous solution. J Phys Chem Ref Data 17(2):513–886
Bielski B, Arudi RL, Sutherland MW (1983) A study of the reactivity of HO2/O2 − with unsaturated fatty acids. J Biol Chem 258(8):4759–4761
Ren X, Sun Y, Fu X, Zhu L, Cui Z (2013) DFT comparison of the OH-initiated degradation mechanisms for five chlorophenoxy herbicides. J Mol Model 19(6):2249–2263
Niu J, Lin H, Gong C, Sun X (2013) Theoretical and experimental insights into the electrochemical mineralization mechanism of perfluorooctanoic acid. Environ Sci Technol 47(24):14341–14349
Zhang C, Sun T, Sun X (2011) Mechanism for OH-initiated degradation of 2,3,7,8-tetrachlorinated dibenzo-p-dioxins in the presence of O2 and NO/H2O. Environ Sci Technol 45(11):4756–4762
Manoj P, Prasanthkumar K, Manoj V, Aravind UK, Manojkumar T, Aravindakumar C (2007) Oxidation of substituted triazines by sulfate radical anion (SO4· −) in aqueous medium: a laser flash photolysis and steady state radiolysis study. J Phys Org Chem 20(2):122–129
Wang Y, Liu Y, Luo Y, Zhang W, Zhong R (2006) Theoretical study on the mechanisms of the reaction of peroxynitrous acid and phenol. Acta Phys Chem Sin 22(10):1266–1272
Ramos B, Farah J, Teixeira A (2012) Estimating reaction constants by ab initio molecular modeling: a study on the oxidation of phenol to catechol and hydroquinone in advanced oxidation processes. Braz J Chem Eng 29(1):113–120
Jayathilaka PB, Pathiraja GC, Bandara A, Subasinghe ND, Nanayakkara N (2014) Theoretical study of phenol and hydroxyl radical reaction mechanism in aqueous medium by the DFT/B3LYP/6-31 + G (d, p)/CPCM model. Can J Chem 92(9):809–813
Xu C, Wang L (2013) Atmospheric oxidation mechanism of phenol initiated by OH radical. J Phys Chem A 117(11):2358–2364
Carrier M, Guillard C, Besson M, Bordes C, Chermette H (2009) Photocatalytic degradation of diuron: experimental analyses and simulation of HO radical attacks by density functional theory calculations. J Phys Chem A 113(22):6365–6374
Batiha M, Al-Muhtaseb AAH, Altarawneh M (2012) Theoretical study on the reaction of the phenoxy radical with O2, OH, and NO2. Int J Quantum Chem 112(3):848–857
Huang Y, Huang Y, Tsai H, Chen H (2010) Degradation of phenol using low concentration of ferric ions by the photo-Fenton process. J Taiwan Inst Chem Eng 41(6):699–704
Bossmann SH, Oliveros E, Göb S, Siegwart S, Dahlen EP, Payawan L, Straub M, Wörner M, Braun AM (1998) New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A 102(28):5542–5550
Bremner DH, Burgess AE, Houllemare D, Namkung K-C (2006) Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl Catal B 63(1):15–19
Zazo J, Casas J, Mohedano A, Gilarranz M, Rodriguez J (2005) Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ Sci Technol 39(23):9295–9302
Kwon BG, Lee DS, Kang N, Yoon J (1999) Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Res 33(9):2110–2118
Mijangos F, Varona F, Villota N (2006) Changes in solution color during phenol oxidation by Fenton reagent. Environ Sci Technol 40(17):5538–5543
Feng J, Hu X, Yue PL (2004) Degradation of salicylic acid by photo-assisted Fenton reaction using Fe ions on strongly acidic ion exchange resin as catalyst. Chem Eng J 100(1):159–165
Grymonpré DR, Sharma AK, Finney WC, Locke BR (2001) The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors. Chem Eng J 82(1):189–207
Bloss C, Wagner V, Jenkin M, Volkamer R, Bloss W, Lee J, Heard D, Wirtz K, Martin-Reviejo M, Rea G (2005) Development of a detailed chemical mechanism (MCMv3. 1) for the atmospheric oxidation of aromatic hydrocarbons. Atmos Chem Phys 5(3):641–664
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103(12):4605–4638
Atkinson R, Carter WP, Darnall KR, Winer AM, Pitts JN (1980) A smog chamber and modeling study of the gas phase NOx–air photooxidation of toluene and the cresols. Int J Chem Kinet 12(11):779–836
Jenkin M, Saunders S, Wagner V, Pilling M (2003) Protocol for the development of the master chemical mechanism, MCM v3 (part B): tropospheric degradation of aromatic volatile organic compounds. Atmos Chem Phys 3(1):181–193
Wagner V, Jenkin M, Saunders S, Stanton J, Wirtz K, Pilling M (2003) Modelling of the photooxidation of toluene: conceptual ideas for validating detailed mechanisms. Atmos Chem Phys 3(1):89–106
Wang H, Li J, Quan X, Wu Y, Li G, Wang F (2007) Formation of hydrogen peroxide and degradation of phenol in synergistic system of pulsed corona discharge combined with TiO2 photocatalysis. J Hazard Mater 141(1):336–343
Ding Z, Lu G, Greenfield P (2000) Role of the crystallite phase of TiO2 in heterogeneous photocatalysis for phenol oxidation in water. J Phys Chem. B 104(19):4815–4820
Serpone N, Maruthamuthu P, Pichat P, Pelizzetti E, Hidaka H (1995) Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: chemical evidence for electron and hole transfer between coupled semiconductors. J Photochem Photobiol, A 85(3):247–255
Doong R, Chen C, Maithreepala R, Chang S (2001) The influence of pH and cadmium sulfide on the photocatalytic degradation of 2-chlorophenol in titanium dioxide suspensions. Water Res 35(12):2873–2880
Scheck CK, Frimmel FH (1995) Degradation of phenol and salicylic acid by ultraviolet radiation/hydrogen peroxide/oxygen. Water Res 29(10):2346–2352
Chitose N, Ueta S, Seino S, Yamamoto TA (2003) Radiolysis of aqueous phenol solutions with nanoparticles. 1. Phenol degradation and TOC removal in solutions containing TiO2 induced by UV, γ-ray and electron beams. Chemosphere 50(8):1007–1013
Nageswara Rao A, Sivasankar B, Sadasivam V (2009) Kinetic study on the photocatalytic degradation of salicylic acid using ZnO catalyst. J Hazard Mater 166(2):1357–1361
Kubesch K, Zona R, Solar S, Gehringer P (2005) Degradation of catechol by ionizing radiation, ozone and the combined process ozone-electron-beam. Radiat Phys Chem 72(4):447–453
Duarte C, Sampa M, Rela P, Oikawa H, Silveira C, Azevedo A (2002) Advanced oxidation process by electron-beam-irradiation-induced decomposition of pollutants in industrial effluents. Radiat Phys Chem 63(3):647–651
Lin K, Cooper WJ, Nickelsen MG, Kurucz CN, Waite TD (1995) Decomposition of aqueous solutions of phenol using high energy electron beam irradiation—a large scale study. Appl Radiat Isot 46(12):1307–1316
Li X, Cui Y, Feng Y, Xie Z, Gu J (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res 39(10):1972–1981
Papouchado L, Sandford R, Petrie G, Adams R (1975) Anodic oxidation pathways of phenolic compounds Part 2. Stepwise electron transfers and coupled hydroxylations. J Electroanal Chem Interfacial Electrochem 65(1):275–284
Comninellis C, Nerini A (1995) Anodic oxidation of phenol in the presence of NaCl for wastewater treatment. J Appl Electrochem 25(1):23–28
Comninellis C, Pulgarin C (1991) Anodic oxidation of phenol for waste water treatment. J Appl Electrochem 21(8):703–708
Tezuka M, Iwasaki M (1998) Plasma induced degradation of chlorophenols in an aqueous solution. Thin Solid Films 316(1):123–127
Yuan M, Watanabe T, Chang C (2010) DC water plasma at atmospheric pressure for the treatment of aqueous phenol. Environ Sci Technol 44(12):4710–4715
Satoh K, Murakami M, Itoh H (2013) Pulsed-plasma degradation of phenol in an aqueous solution. In: 21st international symposium on plasma chemistry (ISPC 21), Cairns Convention Centre, Queensland, Australia
Liu Y, Jiang X (2005) Phenol degradation by a nonpulsed diaphragm glow discharge in an aqueous solution. Environ Sci Technol 39(21):8512–8517
Hoeben W, Van Veldhuizen E, Rutgers W, Cramers C, Kroesen G (2000) The degradation of aqueous phenol solutions by pulsed positive corona discharges. Plasma Sources Sci Technol 9(3):361
Hoeben W, Van Veldhuizen E, Rutgers W, Kroesen G (1999) Gas phase corona discharges for oxidation of phenol in an aqueous solution. J Phys D Appl Phys 32(24):L133
Hoeben WFLM (2000) Pulsed corona-induced degradation of organic materials in water. Ph.D. Dissertation, Technische Universiteit Eindhoven, Eindhoven
Lukes P, Locke BR (2005) Degradation of substituted phenols in a hybrid gas-liquid electrical discharge reactor. Ind Eng Chem Res 44(9):2921–2930
Lukes P (2001) Water treatment by pulsed streamer corona discharge. Ph.D. Thesis, Prague
Li J, Sato M, Ohshima T (2007) Degradation of phenol in water using a gas–liquid phase pulsed discharge plasma reactor. Thin Solid Films 515(9):4283–4288
Hayashi D, Hoeben W, Dooms G, Van Veldhuizen E, Rutgers W, Kroesen G (2000) Influence of gaseous atmosphere on corona-induced degradation of aqueous phenol. J Phys D Appl Phys 33(21):2769
Sano N, Kawashima T, Fujikawa J, Fujimoto T, Kitai T, Kanki T, Toyoda A (2002) Decomposition of organic compounds in water by direct contact of gas corona discharge: influence of discharge conditions. Ind Eng Chem Res 41(24):5906–5911
Sharma A, Locke B, Arce P, Finney W (1993) A preliminary study of pulsed streamer corona discharge for the degradation of phenol in aqueous solutions. Hazard Waste Hazard Mater 10(2):209–219
Sugiarto AT, Sato M (2001) Pulsed plasma processing of organic compounds in aqueous solution. Thin Solid Films 386(2):295–299
Tothova I, Lukes P, Clupek M, Babicky V, Janda V (2009) Removal of nonylphenol by pulsed corona discharge in water. In: 19th international symposium on plasma chemistry, Bochum
Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G, Nakatsuji H, Caricato M, Li X (2009) Gaussian 09, Revision D. 01. Gaussian Inc., Wallingford, CT
Ditchfield R, Hehre W, Pople JA (1971) Self-consistent molecular-orbital methods. 9. extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54(2):724–728
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1–3):215–241
Tomasi J, Mennucci B, Cances E (1999) The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J Mol Struct 464(1):211–226
Neese F (2012) The ORCA program system. WIREs Comput Mol Sci 2(1):73–78
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. PCCP 13(14):6670–6688
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. PCCP 7(18):3297–3305
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396
Goerigk L, Grimme S (2010) Efficient and accurate double-hybrid-meta-GGA density functionals· evaluation with the extended GMTKN30 database for general main group thermochemistry, kinetics, and noncovalent interactions. J Chem Theory Comput 7(2):291–309
Marenich AV, Ho J, Coote ML, Cramer CJ, Truhlar DG (2014) Computational electrochemistry: prediction of liquid-phase reduction potentials. PCCP 16(29):15068–15106
Li X, Frisch MJ (2006) Energy-represented direct inversion in the iterative subspace within a hybrid geometry optimization method. J Chem Theory Comput 2(3):835–839
Peng C, Bernhard Schlegel H (1993) Combining synchronous transit and quasi-newton methods to find transition states. Isr J Chem 33(4):449–454
Wigner EP (1997) Über das Überschreiten von Potentialschwellen bei chemischen Reaktionen. In: Wightman A (ed) Part I: physical chemistry. Part II: solid state physics, vol 4. Springer, Berlin, pp 96–109
Wirz J (2010) Kinetic studies of keto-enol and other tautomeric equilibria by flash photolysis. Adv Phys Org Chem 44:325
Gao J, Liu Y, Yang W, Pu L, Yu J, Lu Q (2003) Oxidative degradation of phenol in aqueous electrolyte induced by plasma from a direct glow discharge. Plasma Sources Sci Technol 12(4):533
Bordwell FG, Cheng J (1991) Substituent effects on the stabilities of phenoxyl radicals and the acidities of phenoxyl radical cations. J Am Chem Soc 113(5):1736–1743
Knispel R, Koch R, Siese M, Zetzsch C (1990) Adduct formation of OH radicals with benzene, toluene, and phenol and consecutive reactions of the adducts with NOx and O2. Ber Bunsenges Phys Chem 94(11):1375–1379
Sherrill CD (2005) Chapter 4: Bond breaking in quantum chemistry. In: Annual reports in computational chemistry, vol 1. Elsevier, Amsterdam, pp 45–56
McFerrin CA, Hall RW, Dellinger B (2008) Ab initio study of the formation and degradation reactions of semiquinone and phenoxyl radicals. J Mol Struct 848(1):16–23
Hunter EP, Desrosiers MF, Simic MG (1989) The effect of oxygen, antioxidants, and superoxide radical on tyrosine phenoxyl radical dimerization. Free Radic Biol Med 6(6):581–585
Weiss R (1970) The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res Oceanogr Abstr 17:721–735
Kanazawa S, Kawano H, Watanabe S, Furuki T, Akamine S, Ichiki R, Ohkubo T, Kocik M, Mizeraczyk J (2011) Observation of OH radicals produced by pulsed discharges on the surface of a liquid. Plasma Sources Sci Technol 20(3):034010
Sahni M (2006) Analysis of the chemical reactions in pulsed streamer discharges: an experimental study. Ph.D. Dissertation, Florida State University, Tallahassee
Hu H, Dibble TS (2013) Quantum chemistry, reaction kinetics, and tunneling effects in the reaction of methoxy radicals with O2. J Phys Chem A 117(51):14230–14242
Nguyen HMT, Peeters J, Nguyen MT, Chandra AK (2004) Use of DFT-based reactivity descriptors for rationalizing radical reactions: a critical analysis. J Phys Chem A 108(3):484–489
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
DeMatteo MP, Poole JS, Shi X, Sachdeva R, Hatcher PG, Hadad CM, Platz MS (2005) On the electrophilicity of hydroxyl radical: a laser flash photolysis and computational study. J Am Chem Soc 127(19):7094–7109
Bachrach SM (2014) Computational organic chemistry. Wiley, New York
Sawaki Y, Foote CS (1983) Mechanism of carbon-carbon cleavage of cyclic 1, 2-diketones with alkaline hydrogen peroxide. The acyclic mechanism and its application to the basic autoxidation of pyrogallol. J Am Chem Soc 105(15):5035–5040
Wheast R (1984) Handbook of chemistry and physics. CRC Press, Boca Raton
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys 300(2):535–543
Atkins P (1998) Physical Chemistry, 6th edn. Oxford University Press, Oxford
Marcus RA (1956) On the theory of oxidation-reduction reactions involving electron transfer. I J Chem Phys 24(5):966–978
Raghavan N, Steenken S (1980) Electrophilic reaction of the hydroxyl radical with phenol. Determination of the distribution of isomeric dihydroxycyclohexadienyl radicals. J Am Chem Soc 102(10):3495–3499
Acknowledgements
One of the authors (S. Mededovic Thagard) would like to acknowledge the support of the National Science Foundation (CBET: #1336385).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, X., McLaughlin, J.B., Melman, A. et al. Quantum Chemical Approach for Determining Degradation Pathways of Phenol by Electrical Discharge Plasmas. Plasma Chem Plasma Process 37, 5–28 (2017). https://doi.org/10.1007/s11090-016-9758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9758-6