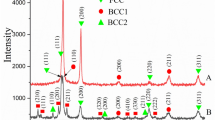
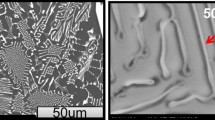
Abstract
The isothermal oxidation behavior of three alumina forming austenitic (AFA) stainless steels with varying composition was studied at 650 and 800 °C in dry air and gases which contained water vapor. The AFA alloys exhibited better oxidation resistance than a “good chromia former” at 650 °C, particularly in H2O-containing atmospheres by virtue of alumina-scale formation. Although the AFA alloys were more resistant than chromia formers, their oxidation resistance was degraded at 650 °C in the presence of water vapor. In dry air the AFA alloys formed, thin continuous alumina scales, whereas in Ar–4%H2–3%H2O the areas of continuous alumina were reduced and Fe oxide-rich nodules and regions of Cr, Mn-rich oxides formed. In some regions internal oxidation of the aluminum occurred in the H2O-containing gas. The alloy OC8 had slightly better resistance than OC4 or OC5 in this atmosphere. The alumina-forming capability of the AFA alloys decreases with increasing temperature and, at 800 °C, they are borderline alumina formers, even in dry air. The oxidation resistance of all three alloys was degraded at 800 °C in atmospheres, which contained water vapor (Air–10%H2O, Ar–3%H2O and Ar–4%H2–3%H2O). The areas, which formed continuous alumina, were reduced in these atmospheres and areas of internal oxidation occurred. However, as a result of the borderline alumina-forming capability of the AFA alloys it was not possible to determine which of the H2O-containing atmospheres was more severe or to rank the alloys in terms of their performance. The experimental results indicate that the initial microstructure of the AFA alloys also plays a role in their oxidation performance. Less protective oxides formed at 800 °C when alloy OC8 was equilibrated before exposure rather than being exposed in the as-processed condition. The reason for this is the presence of different phases in the bulk of the two specimens.


























Similar content being viewed by others
References
Y. Yamamoto, M. P. Brady, Z. P. Lu, P. J. Maziasz, C. T. Liu, B. A. Pint, K. L. More, H. M. Meyer and E. A. Payzant, Science 316, 2007 (433–436).
M. P. Brady, Y. Yamamoto, M. L. Santella, P. J. Maziasz, B. A. Pint, C. T. Liu, Z. P. Lu and H. Bei, JOM 60, 2008 (12–18).
Y. Yamamoto, M. L. Santella, M. P. Brady, H. Bei and P. J. Maziasz, Metallurgical and Materials Transactions A 40, 2009 (1868–1880).
Y. Yamamoto, M. P. Brady, Z. P. Lu, C. T. Liu, M. Takeyama, P. J. Maziasz and B. A. Pint, Metallurgical and Materials Transactions A 38, 2007 (2737–2746).
M. P. Brady, Y. Yamamoto, M. L. Santella and L. R. Walker, Oxidation of Metals 72, 2009 (311–333).
M. P. Brady, Y. Yamamoto, M. L. Santella and B. A. Pint, Scripta Materialia 57, 2007 (1117–1120).
Y. Yamamoto, M. Takeyama, Z. P. Lu, C. T. Liu, N. D. Evan, P. J. Maziasz and M. P. Brady, Intermetallics 16, 2008 (453–462).
G. Stein-Brzozowska, J. Maier and G. Scheffknecht, Energy Procedia 4, 2011 (2035–2042).
A. U. Syed, N. J. Simms and J. E. Oakey, Fuel 101, 2012 (62–73).
H. Asteman, J.-E. Svensson and L.-G. Johansson, Corrossion Science 44, 2002 (2635–2649).
H. Asteman, J.-E. Svensson and L.-G. Johansson, Oxidation of Metals 57, 2002 (193–216).
J. E. Segerdahl, J.-E. Svensson and L.-G. Johansson, Materials and Corrossion 53, 2002 (247–255).
C. Wagner, Corrosion Science 5, 1965 (751–764).
N. Birks, G. H. Meier and F. S. Pettit, Introduction to the High Temperature Oxidation of Metals, 2nd ed, (Cambridge University Press, New York, 2006).
P. J. Maziasz, JOM 41, 1989 (14–20).
M. P. Brady, K. A. Unocic, M. J. Lance, M. L. Santella, Y. Yamamoto and L. R. Walker, Oxidation of Metals 75, 2011 (337–357).
M. P. Brady, J. H. Magee, Y. Yamamoto, P. J. Maziasz, M. L. Santella, B. A. Pint, and H. Bei, Development and Exploratory Scale-Up of Alumina-Forming Austenitic (AFA) Stainless Steels. Proceedings of Stainless Steel World 2009 Conference & Expo, Maastricht, The Netherlands, November 10–12, 2009, Stainless Steel World, The Netherlands, 2009.
M. P. Brady, J. Magee, Y. Yamamoto, D. Helmick and L. Wang, Materials Science and Engineering: A 590, 2014 (101–115).
N. Mu, K. Y. Jung, N. M. Yanar, G. H. Meier, F. S. Pettit and G. R. Holcomb, Oxidation of Metals 78, 2012 (221–237).
V. K. Tolpygo and D. R. Clarke, Materials at High Temperatures 17, 2000 (59–70).
D. M. Lipkin and D. R. Clarke, Oxidation of Metals 45, (3/4), 1996 (267–280).
Q. Wen, D. M. Lipkin and D. R. Clarke, Journal of American Ceramic Society 81, (12), 1998 (3345–3348).
E. Essuman, G. H. Meier, J. Zurek, M. Hänsel, L. Singheiser and W. J. Quadakkers, Scripta Materialia 57, 2007 (845–848).
L. Niewolak, D. J. Young, H. Hattendorf, L. Singheiser and W. J. Quadakkers, Oxidation of Metals 82, 2014 (123–143).
C. Wagner, Journal of the Electrochemical Society 99, 1952 (369–380).
Acknowledgments
The authors gratefully acknowledge the Office of Naval Research for the financial support of this work under Contract N00014-12-1-0612, Dr. Airan Perez, Scientific Monitor. Oak Ridge National Laboratory and Carpenter Technology Corporation are acknowledged for providing the AFA alloys for this study. Dr. L. Niewolak, Forschungszentrum, Juelich, Germany, is acknowledged for characterization of the microstructure of the OC8 creep specimen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Research Involving Human participants and/or Animals and Informed Consent
There were no human participants or animals in this study.
Rights and permissions
About this article
Cite this article
Yanar, N.M., Lutz, B.S., Garcia-Fresnillo, L. et al. The Effects of Water Vapor on the Oxidation Behavior of Alumina Forming Austenitic Stainless Steels. Oxid Met 84, 541–565 (2015). https://doi.org/10.1007/s11085-015-9581-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-015-9581-0