Abstract
Oxygenic photosynthesis is the most important bioenergetic event in the history of our planet—it evolved once within the Cyanobacteria, and remained largely unchanged as it was transferred to algae and plants via endosymbiosis. Manganese plays a fundamental role in this history because it lends the critical redox behavior of the water-oxidizing complex of photosystem II. Constraints from the photoassembly of the Mn-bearing water-oxidizing complex fuel the hypothesis that Mn(II) once played a key role as an electron donor for anoxygenic photosynthesis prior to the evolution of oxygenic photosynthesis. Here we review the growing body of geological and geochemical evidence from the Archean and Paleoproterozoic sedimentary records that supports this idea and demonstrates that the oxidative branch of the Mn cycle switched on prior to the rise of oxygen. This Mn-oxidizing phototrophy hypothesis also receives support from the biological record of extant phototrophs, and can be made more explicit by leveraging constraints from structural biology and biochemistry of photosystem II in Cyanobacteria. These observations highlight that water-splitting in photosystem II evolved independently from a homodimeric ancestral type II reaction center capable of high potential photosynthesis and Mn(II) oxidation, which is required by the presence of homologous redox-active tyrosines in the modern heterodimer. The ancestral homodimer reaction center also evolved a C-terminal extension that sterically precluded standard phototrophic electron donors like cytochrome c, cupredoxins, or high-potential iron-sulfur proteins, and could only complete direct oxidation of small molecules like Mn2+, and ultimately water.
Similar content being viewed by others
Introduction
After the development of life itself, the evolution of oxygenic photosynthesis is the most fundamental and transformative event in the history of our planet—and manganese plays a special role in this history. The most important biochemical innovation to enable the phototrophic splitting of water and the production of molecular oxygen is the water-oxidizing complex (WOC) of photosystem II (PSII). The WOC is a high-valent cubane cluster of four redox-active Mn centers and a Ca center, bound by oxo-bridges (McEvoy and Brudvig 2006, Umena et al. 2011). And it serves an important function of acting as a type of redox capacitor that adapts the native single electron chemistry of the reaction center for the four-electron oxidation of two water molecules.
How did the WOC originate? In considering the evolutionary history of photosynthesis, Olson (1970) provided the necessary realization that some transitional state came before PSII, and that this ancestral protein would have had some meaningful but different physiology than the water-splitting reactions that characterize PSII. In evolution, the process of exaptation (Gould and Vrba 1982) is a commonly held and useful concept wherein pre-existing structures are recruited and adapted for novel functions. However, compared to other metals like iron, there are only a few protein families that bear Mn cofactors. Blankenship and Hartman (1998) suggested that the WOC might have derived from catalase (which has a binuclear Mn center and performs chemistry with high-valent oxygen species), but sequence and structural data collected since then has not born this out. Indeed one of the truly remarkable features of the WOC is that it is an evolutionary singularity, and shares no meaningful similarity with anything else in the natural protein world.
Photoassembly of the WOC complex provides important insights into WOC evolution. This biosynthetic process demands only Mn2+ and light (Tamura and Cheniae 1987); during catalytic turnover and electron transport through the photosystem five electrons are donated from four manganese atoms to produce the basal oxidation state (S0; Mn(III)Mn(IV)3) of the WOC (McEvoy and Brudvig 2006). It is critical to note that all water-splitting first begins with manganese oxidation, and that these electrons derived from manganese are indistinguishable from those later provided by the oxidation of water. This phenomenon highlights a class of ontogeny-recapitulates-phylogeny hypotheses for the WOC, wherein Mn(II) played a role as an electron donor for phototrophy by ancestral anoxygenic phototrophic Cyanobacteria prior to oxygenic photosynthesis (Zubay 1996, Dismukes et al. 2001, Allen and Martin 2007, Johnson et al. 2013a, b). This idea is particularly valuable because it provides clear expectations that can be tested against observations of the geological record. Recently we provided the first strong support for this hypothesis with detailed geological and geochemical data from early Paleoproterozoic strata in South Africa (Johnson et al. 2013a). Below we will briefly review those results, discuss some new ones from other geologic settings, and then develop a more detailed hypothesis for how ancestral PSII might have proceeded from Mn oxidation to water splitting by leveraging observations and constraints from biochemistry and structural biology with evolutionary theory.
Observations from the Geologic Record
Manganese is the third most abundant transition metal in Earth’s crust, where it is present only as Mn(II) and substitutes for ferrous iron in a wide variety of primary igneous minerals (Turekian and Wedepohl 1961). Rock weathering, therefore, provides a substantial flux of Mn2+ to Earth surface waters. Mn2+ is also soluble unless oxidized to Mn(III) or Mn(IV) which subsequently undergoes hydrolysis and forms insoluble oxide phases that rapidly sediment (Stumm and Morgan 1996). Compared to iron or sulfur, however, manganese requires high potential oxidants (like O2 or species derived from O2) to undergo redox cycling. In addition, the oxidation of Mn(II) is comparatively sluggish even in environments with abundant O2, particularly in the absence of biological catalysts (Morgan 2005). This forms the logic for a geologic test of the Mn-phototrophy hypothesis for the origin of the WOC derived above: if correct, Mn-oxidation should have occurred prior to the evolution of oxygenic photosynthesis and in the (effective) absence of molecular oxygen or other oxidants thereby derived.
Throughout Archean time (>2.5 Ga), seawater (both surface and deep) contained elevated levels of Mn2+ (Fischer and Knoll 2009). This inference is supported by a substantial body of observations of the chemistry of Archean carbonates (Veizer et al. 1989a, Veizer et al. 1989b, Veizer 1985, Ronov and Migdisov 1971, Beukes 1987, Sumner 1997, Sumner and Grotzinger 1996, Sumner and Grotzinger 2004), and shows no Mn oxidation with the only sink for Mn(II) derived from rock weathering as a trace constituent of CaCO3 minerals precipitated on carbonate platforms. The same was not true for iron and sulfur, which had already developed complex redox cycles by that time (e.g., - Fischer and Knoll 2009, Fischer et al. 2014). Furthermore, iron—the largest pool of available donors and acceptors before water-splitting—was effectively removed from surface seawater by phototrophs suggesting that autotrophs living in surface seawater during Archean time were electron-limited (Beukes 1987, Fischer and Knoll 2009, Kappler et al. 2005, Kharecha et al. 2005). These observations are important because they provide an additional answer for the question “Why did nature choose manganese” for water-splitting (Armstrong 2008). The presence of Mn2+ in surface seawater would have provided an evolutionary opportunity for those anoxygenic phototrophs, however in order to oxidze Mn(II) they would need to develop high potential photosynthesis to exploit the resource—an evolutionary path that would end in water oxidation (Johnson et al. 2013a).
Confirming a historical prediction of the Mn-phototrophy hypothesis, the earliest authigenic Mn deposits (reflecting Mn oxidation and sedimentary concentration of oxides) occur shortly before the rise of O2, in the ~2.415 Ga Koegas Subgroup preserved in the Griqualand West structural sub-basin of the Kaapvaal Craton of South Africa (Schröder et al. 2011, Johnson et al. 2013a). These strata are comprised of near-shore marine deltaic deposits on the western margin of the Kaapvaal craton that accumulated horizons of Mn-rich iron formation tied to lobe-switching on the delta. Independent observations of mass anomalous fractionation of multiple sulfur isotope ratios in authigenic pyrite coupled with the widespread occurrence of detrital pyrite demonstrated that O2 levels remained far too low to explain the observed Mn-rich sediments (Farquhar et al. 2000, Pavlov and Kasting 2002). With subsequent work we also observed detrital uraninite (a redox-sensitive U-bearing phase) in this sequence and developed a kinetic sediment transport model that combines chemical and physical weathering to quantitatively estimate environmental O2 levels in Earth surface environments at that time (Johnson et al. 2014). The redox sensitive detrital grains in the Koegas Subgroup show no oxidation on their path from bedrock to soils and hillslopes, channels, bars, and floodplains, ultimately to their depocenter in the basin. This provides an unambiguous and well-understood redox proxy that constrains O2 at that time to extremely low levels—concentrations many orders of magnitude too small to explain the Mn-enrichments (Johnson et al. 2013a, 2013b). Altogether this ecosystem-scale paleoenvironmental evidence strongly suggests that Mn2+ was being used by anoxygenic phototrophs as an electron donor prior to the evolution of oxygenic photosynthesis and rise of oxygen at ~2.3 Ga.
A global test of this hypothesis is still hindered by the general scarcity of well-preserved and sufficiently studied sedimentary basins of early Paleoproterozoic age, however several broadly coeval deposits exist that support this idea. Turee Creek strata from Western Australia of broad similar age to the Koegas deposits contain a thin horizon enriched in Mn, though the paleoenvironments and processes of Mn-mineralization aren’t as well constrained at present (Williford et al. 2011). Another thin horizon in the middle of the Huronian Supergroup (Sekine et al. 2011) also contains Mn-enrichments possibly deposited prior to the rise of O2 (Kopp et al. 2005). Earlier deposits in the ~2.9 Ga Witswatersrand and Mozaan basins of South Africa contain horizons in the stratigraphy, including shales and iron formations, that contain levels of manganese (Smith 2007, Planavsky et al. 2014) elevated relative to those seen throughout other Archean successions (Klein 2005). These basins also contain abundant detrital pyrite and uraninite (e.g., Frimmel 2005) and sulfur isotope mass anomalies (Ono et al. 2006, Guy et al. 2012) that show extremely low free environmental oxygen at that time. But while it’s tempting to draw support for Mn-phototrophy deeper into Archean time, more detailed work is required to untangle the complex petrogenesis and multiple phases of mineralization present in these metamorphic rocks before any robust conclusions can be drawn about paleoenvironmental redox processes operating in the Witswatersrand and Mozaan basins (Smith 2007).
So a growing set of observations from the geological record support the notion of a Mn-oxidizing transitional state during the evolution of oxygenic photosynthesis. However these observations only offer a broad brush ecosystem-scale paleoenvironmental view on this process. To arrive at a deeper understanding of how ancestral photosynthesis evolved from single electron oxidation reactions to the four electron splitting of water, we need to draw on observations and constraints by structural biology, biochemistry, and genomics.
Additional Constraints from Biochemistry and Structural Biology
From electron transfer mechanics, highly conserved structural features, and phylogenetic analyses it is clear that PSII is most closely related to RCII, and that PSII and RCII evolved to become a heterodimeric reaction centers independently (Sadekar et al. 2006). So one can infer that the ancestor of RCII and PSII was an anoxygenic type II reaction center (e.g., cytochrome c - quinone oxidoreductase) homodimer that received electrons from single electron carrier small soluble proteins (like cytochrome c, cupredoxins, and high potential iron-sulfur proteins). It produced low potential oxidants (<~400 mV) as evidenced by the wide diversity of extant anoxygenic phototrophs with distal electron donors of ferrous iron, reduced S compounds, organic compounds, or molecular hydrogen in addition to cyclic electron transport (Hohmann-Marriott and Blankenship 2011). We also infer that it reduced menaquinones (Schoepp-Cothenet et al. 2009) from evolutionary analysis of the distribution of “high-potential” quinones currently in nature. From the Granick hypothesis (and observations of pigments in RCI) it is also reasonable to infer that it contained chlorophyll a (Granick 1957, Bryant and Liu 2013).
The transition from anoxygenic to oxygenic phototrophy was a complex process that required the acquisition of three unique biochemical attributes: 1) a photosystem capable of oxidizing high-potential (> +820 mV) electron donors, 2) a bioinorganic complex that could catalyze the oxidiation H2O to O2 (the WOC), 3) and the coupling of two photosystems in series to generate low potential electron donors (ferrodoxin and NADPH) capable of readily fixing CO2 into biomass. It is possible to ordinate the evolution of these features since the origin of the WOC required the presence of a pre-existing high-potential photosystem to provide the oxidizing potential to split water. Furthermore the CP43 subunit of PSII, which evolved from a duplication of the N-terminus of PSI (Mix et al. 2005), provides ligands for the WOC. This strongly suggests some type of high-potential anoxygenic phototrophy that utilized two photosystems was a direct precursor to oxygenic phototrophy.
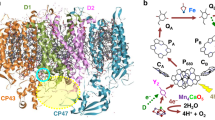
A number of powerful evolutionary insights can be gained from structural observations of the PSII heterodimer composed of the D1 and D2 proteins. Features shared by both sides of the protein can be confidently assigned to the ancestral state, and would have contributed to its catalytic characteristics. Redox-active tyrosines are present at homologous positions in the heterodimer. These residues demonstrate that the homodimeric ancestor received electrons from both sides of the protein and must have been capable of generating high potential oxidants—far higher than required for typical anoxygenic photosynthesis but in line with the requirements for manganese and water oxidation (Rutherford and Faller 2002). Furthermore a homologous C-terminal extension consisting of a loop (likely caused by a read-through) exists on the periplasmic side of both D1 and D2 that makes it biologically impossible to feed electrons from standard phototrophic electron donors like cytochrome c, cupredoxins, or high-potential iron-sulfur proteins to P680+, because the distances would be too large for effective electron transfer (>25 Å). This requires that the ancestral homodimer could only oxidize small molecules like Mn2+, and ultimately water via the WOC, directly. We hypothesize that prior to heterodimerism, the ancestral PSII only oxidized manganese and did not yet form a fully functional WOC because 1) the WOC is only present on one side of the extant reaction center, and 2) two functional WOCs—which would be required by a homodimeric ancestral PSII capable of water oxidation—would have to coordinate the transfer of eight electrons to oxidize water on both sides of the protein complex (Fig. 1). This is evolutionarily unpalatable as coordinating four electron transfers is already sufficiently taxing. However Mn-oxidation by both sides of the protein complex is viable and might have some advantages in being able to rapidly provide electrons to the photosystem. For this reason, we view the evolution of heterodimerism in PSII as effectively synchronous with the origin of the water oxidizing complex, as it is unclear how to break this symmetry at present.
Schematic showing steps in the evolution of water-splitting by photosystem II according to constraints from structural biology of PS(II). Reaction centers are shown as a dimer. Pathways of electron flow are marked with gray arrows. Thick green lines show chlorophyll pigments. MQ menaquinol, PQ plastoquinol, cytC cytochrome c or similar small protein single electron carrier. Relative time moves left to right. The center panel describes the electron transfer path most consistent with observations of Mn-phototrophy from the geologic record (Johnson et al. 2013a, b) and molecular evolution (e.g., Sadekar et al. 2006). Rightmost panel describes the electron transfer path in the extant PSII heterodimer found in oxygenic Cyanobacteria
References
Allen JF, Martin W (2007) Evolutionary biology: out of thin air. Nature 445:610–612
Armstrong FA (2008) Why did Nature choose manganese to make oxygen? Philos Trans R Soc B 363:1263–1270
Beukes NJ (1987) Facies relations, depositional environments, and diagenesis in a major early Proterozoic stromatolitic carbonate platform to basinal sequence, Campbellrand Subgroup, Transvaal Supergroup, Southern Africa. Sediment Geol 54:1–46
Blankenship RE, Hartman H (1998) The origin and evolution of oxygenic photosynthesis. Trends Biochem Sci 23:94–97
Bryant ZA, Liu Z (2013) Green bacteria: insights into green bacterial evolution through genomic analyses, advances in botanical research. 66, Elsevier Ltd. ISSN 0065–2296, http://dx.doi.org/10.1016/B978-0-12-397923-0.00004-7
Dismukes GC, Klimov VV, Baranov SV, Kozlov YN, DasGupta J, Tryshkin A (2001) The origin of atmospheric oxygen on earth: the innovation of oxygenic photosynthesis. Proc Natl Acad Sci U S A 98:2170–2175
Farquhar J, Bao HM, Thiemens M (2000) Atmospheric influence of Earth’s earliest sulfur cycle. Science 289:756–758
Fischer WW, Knoll AH (2009) An iron shuttle for deepwater silica in Late Archean and early Paleoproterozoic iron formation. Geol Soc Am Bull 121:222–235
Fischer WW, Fike DA, Johnson JE, Raub TD, Guan Y, Kirschvink JL, Eiler JM (2014) SQUID-SIMS is a useful approach to uncover primary signals in the Archean sulfur cycle. Proc Natl Acad Sci 111:5468–5473
Frimmel HE (2005) Archaean atmospheric evolution: evidence from the Witwatersrand gold fields. S Afr Earth Sci Rev 70:1–46
Gould SJ, Vrba ES (1982) Exaptation - a missing term in the science of form. Paleobiology 8:4–15
Granick S (1957) Speculations on the origins and evolution of photosynthesis. Ann N Y Acad Sci 69:292–308
Guy BM, Ono S, Gutzmer J, Kaufman AJ, Lin Y, Fogel ML, Beukes NJ (2012) A multiple sulfur and organic carbon isotope record from conglomeratic sedimentary rocks of the Mesoarchean Witwatersrand Supergroup. S Afr Precambrian Res 219:208–231
Hohmann-Marriott MF, Blankenship RE (2011) The evolution of photosynthesis. Annu Rev Plant Biol 62:515–548
Johnson JE, Webb SM, Thomas K, Ono S, Kirschvink JL, Fischer WW (2013a) Manganese-oxidizing photosynthesis before the rise of cyanobacteria. Proc Natl Acad Sci 108:11238–11243
Johnson JE, Webb SM, Thomas K, Ono S, Kirschvink JL, Fischer WW (2013b) Correcting mistaken views of sedimentary geology, Mn-oxidation rates, and molecular clocks. Proc Natl Acad Sci 110:E4119–E41120
Johnson JE, Gerpheide A, Lamb MP, Fischer WW (2014) O2 constraints from Paleoproterozoic detrital pyrite and uraninite. Geol Soc Am Bull. Published online ahead of print on 27 Feb. 2014, doi: 10.1130/B30949.1
Kappler A, Pasquero C, Konhauser KO, Newman DK (2005) Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria. Geology 33:865–868
Kharecha P, Kasting J, Siefert J (2005) A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology 3:53–76
Klein C (2005) Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am Mineral 90:1473–1499. doi:10.2138/am.2005.1871
Kopp RE, Kirschvink JL, Hilburn IA, Nash CZ (2005) Was the Paleoproterozoic Snowball Earth a biologically-triggered climate disaster? Proc Natl Acad Sci 102:11131–11136
McEvoy JP, Brudvig GW (2006) Water-splitting chemistry of photosystem II. Chem Rev 106:4455–4483
Mix LJ, Haig D, Cavanaugh CM (2005) Phylogenetic analyses of the core antenna domain: investigating the origin of photosystem I. J Mol Evol 60:153–163
Morgan JJ (2005) Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim Cosmochim Acta 69:35–48
Olson JM (1970) The evolution of photosynthesis. Science 168:438–446
Ono S, Beukes NJ, Rumble D, Fogel M (2006) Early evolution of Earth’s atmospheric oxygen from multiple sulfur and carbon isotope records of the 2.9 Ga Pongola Supergroup, Southern Africa. S Afr J Geol 109:97A108
Pavlov AA, Kasting JF (2002) Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology 2:27–41
Planavsky NJ, Asael D, Hofmann A, Reinhard CT, Lalonde SV, Knudsen A, Wang X, Ossa Ossa F, Pecoits E, Smith AJB, Beukes NJ, Bekker A, Johnson TM, Konhauser KO, Lyons TW, Rouxel OJ (2014) Evidence for oxygenic photosynthesis half a billion years before the great oxidation event. Nat Geosci 7:283–286
Ronov AB, Migdisov AA (1971) Evolution of the chemical composition of the rocks in the shields and sediment cover of the Russian and North American Platforms. Sedimentology 16:137–185
Rutherford W, Faller P (2002) Photosystem II: evolutionary perspectives. Philos Trans R Soc London B 358:245–253
Sadekar S, Raymond R, Blankenship RE (2006) Conservation of distantly related membrane proteins: photosynthetic reaction centers share a common structural core. Mol Biol Evol 23:2001–2007
Schoepp-Cothenet B, Lieutaud C, Baymann F, Vermeglio A, Friedrich T, Kramer DM, Nitschke W (2009) Menaquinone as pool quinone in a purple bacterium. Proc Natl Acad Sci 106:8549–8554
Schröder C, Bedorf D, Beukes NJ, Gutzmer J, (2011) From BIF to red beds: Sedimentology and sequence stratigraphy of the Paleoproterozoic Koegas Subgroup (South Africa). Sediment Geol 236(1–2):25–44. doi:10.1016/j.sedgeo.2010.11.007
Sekine Y, Tajika E, Tada R, Hirai T, Goto KT, Kuwatani T, Goto K, Yamamoto S, Tachibana S, Isozaki Y, Kirschvink JL (2011) Manganese enrichment in the Gowganda Formation of the Huronian Supergroup: a highly oxidizing shallow-marine environment after the last Huronian glaciation. Earth Planet Sci Lett 307:201–210
Smith AJB (2007) The Paleoenvironmental Significance of the Iron-formations and Iron-rich Mudstones of the Mesoarchean Witwatersrand-Mozaan Basin, South Africa. UJ Thesis, https://ujdigispace.uj.ac.za/handle/10210/2440
Stumm W, Morgan JJ (1996) Aquatic chemistry: Chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York, p 1042
Sumner DY (1997) Carbonate precipitation and oxygen stratification in Late Archean seawater as deduced from facies and stratigraphy of the Gamohaan and Frisco Formations, Transvaal Supergroup, South Africa. Am J Sci 297:455–487
Sumner DY, Grotzinger JP (1996) Were kinetics of Archean calcium carbonate precipitation related to oxygen concentration? Geology 24:119–122
Sumner DY, Grotzinger JP (2004) Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform. S Afr Sedimentol 51:1273–1299
Tamura N, Cheniae G (1987) Photoactivation of the water-oxidizing complex inPhotosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical andkinetic characterization. Biochim Biophys Acta Bioenerg 890:179–194
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen- evolving photosystem II at a resolution of 1.9 A. Nature 473:55–60
Veizer J (1985) Carbonates and ancient oceans: Isotopic and chemical record on time scales of 107–109 years. In E. T. Sunquist and W. S. Broecker (eds.) The carbon cycle and atmospheric CO2: Natural variations archean to present. Geophysical Monograph Series, 32, 595–601, AGU.
Veizer J, Hoefs J, Ridler RH, Jensen LS, Lowe DR (1989a) Geochemistry of Precambrian carbonates: I. Archean hydrothermal systems. Geochim Cosmochim Acta 53:845–857
Veizer J, Hoefs J, Lowe DR, Thurston PC (1989b) Geochemistry of Precambrian carbonates: II. Archean greenstone belts and Archean sea water. Geochim Cosmochim Acta 53:859–871
Williford KH, Van Kranendonk MJ, Ushikubo T, Kozdon R, Valley JW (2011) Constraining atmospheric oxygen and seawater sulfate concentrations during Paleoproterozoic glaciation: in situ sulfur three-isotope microanalysis of pyrite from the Turee Creek Group, Western Australia. Geochim Cosmochim Acta 75:5686–5705
Zubay G (1996) Origins of life on the earth and in the cosmos, 2nd edn. Academic, San Diego
Acknowledgments
We extend our sincere thanks to Joe Kirschvink, Yuichiro Ueno, Jim Cleaves, and others from the Earth-Life Science Institute for their support and organization of the 2nd International ELSI symposium where we presented this work. Funding for this work was graciously provided by the Agouron Institute (WWF and JH), Packard Foundation (WWF), National Science Foundation Graduate Research Fellowship Program (JEJ), and the Caltech Center for Environment-Microbe Interactions (WWF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, W.W., Hemp, J. & Johnson, J.E. Manganese and the Evolution of Photosynthesis. Orig Life Evol Biosph 45, 351–357 (2015). https://doi.org/10.1007/s11084-015-9442-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9442-5