Abstract
The action of an electric discharge on reduced gas mixtures such as H2O, CH4 and NH3 (or N2) results in the production of several biologically important organic compounds including amino acids. However, it is now generally held that the early Earth’s atmosphere was likely not reducing, but was dominated by N2 and CO2. The synthesis of organic compounds by the action of electric discharges on neutral gas mixtures has been shown to be much less efficient. We show here that contrary to previous reports, significant amounts of amino acids are produced from neutral gas mixtures. The low yields previously reported appear to be the outcome of oxidation of the organic compounds during hydrolytic workup by nitrite and nitrate produced in the reactions. The yield of amino acids is greatly increased when oxidation inhibitors, such as ferrous iron, are added prior to hydrolysis. Organic synthesis from neutral atmospheres may have depended on the oceanic availability of oxidation inhibitors as well as on the nature of the primitive atmosphere itself. The results reported here suggest that endogenous synthesis from neutral atmospheres may be more important than previously thought.
Similar content being viewed by others
Introduction
Organic compounds supplied by either endogenous or exogenous sources are thought to have been necessary for the origin of life. Electric discharges acting on reduced gas mixtures such as H2O, CH4, H2 and NH3 (or N2) result in the production of several biologically important organic compounds such as amino acids (Miller 1953, 1955), providing support for the heterotrophic hypothesis of the origin of life. Although amino acids and a wide array of other biochemical monomers are readily produced from reduced gas mixtures, the geochemical relevance of these model atmospheres has been questioned (Holland 1962; Abelson 1966). As a result, some researchers now either consider the atmospheric composition used in the Miller–Urey experiment implausible, or suggest that reducing conditions only existed in localized regions such as volcanoes or hydrothermal vents.
It is generally agreed that there was little free oxygen in the early atmosphere, but opinions vary regarding the remainder of the primitive atmosphere’s composition. Estimates range from reducing (CH4 + H2 + NH3 + H2O, or CO2 + H2 + N2) to neutral (CO2 + N2 + H2O). A weakly reducing or neutral atmosphere appears to be more in agreement with most current models for the early Earth. Although there may have been a short lived period early in Earth’s history when the atmosphere was reducing due to accumulation of H2 derived from photodissociation of reduced volcanic species (Tian et al. 2005), this would have been rapidly replaced by a more neutral atmosphere as H2 was lost to space. Most models suggest that the early atmosphere consisted of a weakly reducing mixture of CO2, N2, CO, and H2O, with lesser amounts of H2, SO2, CH4 and H2S.
Since the original Miller–Urey experiment, numerous prebiotic simulations have been conducted and have demonstrated that a large assortment of organic molecules can be synthesized using a variety of gas mixtures and energy sources (Sutherland and Whitfield 1997; Miller 1998). Although Miller and Urey (1959) originally rejected the idea of non-reducing conditions for the primitive atmosphere, a number of experiments were later carried out using CO and CO2 model atmospheres (Schlesinger and Miller 1983; Miyakawa et al. 2002) such as those proposed by Haldane (1928). However, the synthesis of organic compounds by the action of electric discharges on neutral gas mixtures is much less efficient than when reduced model atmospheres are used (Folsome et al. 1981; Schlesinger and Miller 1983). As the gas mixture becomes less reducing (less H2, CH4 or NH3), the yields of organic compounds decrease drastically, with glycine being the only major amino acid synthesized. The presence of methane and ammonia appears to be especially important for the formation of diverse mixtures of amino acids (Schlesinger and Miller 1983; Miyakawa et al. 2002). The main problem in the synthesis of amino acids and other biologically relevant organic compounds with non-reducing atmospheres appears to be the limited amount of hydrogen cyanide that is formed, which is a central intermediate in the Strecker amino acid synthesis and an important precursor for the synthesis of nucleobases (Ferris et al. 1978).
Was the primitive terrestrial atmosphere rich in CO2?
It is now believed that the Earth’s core formed relatively quickly. The removal of reducing potential from the upper mantle led to the majority of the volatiles outgassed by volcanism to be in equilibrium with the more oxidized remaining species. It has been argued that during the Hadean and early Archean a CO2-rich neutral atmosphere, containing other species such as CO and N2, would have generated a significant greenhouse effect that would have prevented the early Earth’s oceans from freezing (Kasting 1993a, b; Kasting and Catling 2003). During this period, there were probably no major continents, and thus little silicate weathering. Because this process is nowadays the most important geological process involved in CO2 removal and storage, it has been suggested that the Earth’s CO2 inventory would have been primarily contained in the atmosphere and oceans. With an early Sun approximately ∼70% less luminous then today (Gough 1981), a steady-state atmosphere containing ∼10 bars of CO2 may have been required to maintain a mean surface temperature above the freezing point of water, in the absence of other greenhouse gases.
Although the high CO2 solution for the faint young sun problem was suggested nearly 25 years ago [see Kasting (1993a) and references therein], recent reconsiderations of the early carbon cycle suggest that before extensive tectonic recycling of crustal sediments became common, most of the carbon on the Earth’s surface would have remained buried in the crust and mantle as carbonates (Sleep and Zahnle 2001). Thus, CO2 may not have been present in the atmosphere at levels adequate to prevent global glaciation, making the presence of other greenhouse gases such as CH4 necessary. Without the greenhouse warming associated with high CO2 levels, near global ocean ice cover may have been common on the early Earth (Bada et al. 1994).
The conclusion that the Earth’s early atmosphere was composed of CO2 (1–100 bar) with ∼1 bar N2 (Kasting and Catling 2003), combined with the observed low yields of amino acids when neutral gas mixtures are sparked, led to the conclusion that endogenous prebiotic synthesis would have been only a minor contributor to the prebiotic organic inventory. This prompted the search for alternative sources of organic compounds, such as hydrothermal vents or exogenous delivery. The perceived problems in the synthesis of organic compounds under neutral atmospheric conditions have also been represented by some as evidence against evolutionary approaches to the study of the origins of life (Wells 2000).
Previous reports on the yields of amino acids in electric discharge experiments with N2 + CO2 were low but not zero (Schlesinger and Miller (1983). The small amount of amino acids formed under neutral conditions prompted our reinvestigation of these results in order to better constrain the amounts and types of amino acids that could be produced. The data presented here suggest that the low yields of amino acids reported previously were the outcome of both the experimental conditions and the analytical procedures used. The low yields of amino acids previously reported were likely the result of oxidation of the organic compounds produced during hydrolytic workup by nitrite and nitrate produced by the electric discharge. We also found that the addition of oxidation inhibitors prior to hydrolysis resulted in the recovery of several hundred times more amino acids than reported previously, suggesting that primitive ocean chemistry may also have been an extremely significant aspect of endogenous organic synthesis.
Material and Methods
Medical grade nitrogen gas (purity >99.99% N2) and industrial grade carbon dioxide (claimed by the manufacturer to contain no more than 10 ppm impurities) were purchased from Airgas (Radnor, PA). The purity of the carbon dioxide and nitrogen was measured by mass spectrometry with a Thermofinnigan DeltaPlus XP isotope ratio mass spectrometer by Dr. Bruce Deck of the SIO Analytical Laboratory. Total hydrocarbon contaminants were measured as methane by the height of the amu-16 ion (CH4) against a standard of 1 ppm methane in helium. Assuming equal electron-impact ionization efficiencies for the two gas environments (CO2 and He), leads to 48 ppm of methane or other hydrocarbons in the carbon dioxide. This number is corrected for the instrument blank at this mass number. No methane or other impurities were detected in the nitrogen
Colorimetric assays were performed with a Hewlett Packard Model 8452A Diode Array Spectrophotometer using 1 cm path length quartz cuvettes. An Hitachi L-6200 Intelligent Pump high performance liquid chromatography (HPLC system) was used to separate and identify amino acids as well as to detect HCN.
All sample dilutions, buffers and reagents were prepared in water that was doubly-distilled in glass. The apparatus used in the experiments was the same one used previously [see Schlesinger and Miller (1983)]. Reactions were run at room temperature (∼25°C).
Ammonium ion concentrations were determined by the method of Solorzano (1969). Approximately 100 μl of sample was diluted to 1 ml by the addition of 0.9 ml water and mixed with 0.5 ml of alcoholic phenol (1 ml liquefied phenol added to 95 ml of 95% ethanol and brought to 100 ml with water). To this mixture is added 0.5 ml of sodium nitroprusside (0.15 g in 200 ml H2O) and 1 ml of oxidizing solution (1 ml of 6% (w/v) aqueous sodium hypochlorite in 50 ml of sodium citrate buffer (7.6 g trisodium citrate and 0.4 g sodium hydroxide in 500 ml double distilled water). The final reaction mixture is incubated at room temperature for 1 to 3 h and the resulting blue color is measured at 640 nm. The alcoholic phenol, sodium nitroprusside and oxidizing solutions are made up fresh each day. The range is approximately 10−5 to 10−3 M \({\text{NH}}_4^ + \) as ammonium chloride.
Urea was determined by Annino and Giese’s (1976) modification of the reaction of diacetyl monoxime (DAM) to form a colored diazine derivative in the presence of thiosemicarbazide (TSC) according to Evans (1968). This assay utilizes reagents prepared from stock solutions of acidic ferric chloride (0.25 g FeCl3, 40 ml conc. H2SO4, and 5 ml 85% H3PO4 in 500 ml H20), DAM (2.5 g of DAM in 100 ml H2O), and TSC (0.25 g TSC in 100 ml H2O). DAM-TSC reagent was prepared by mixing 24 ml DAM stock and 10 ml TSC stock and diluting to 100 ml with water. The assay is run by adding 200 μl of sample to 2 ml of Color Reagent, which is made by mixing 40 ml acidic ferric chloride stock with 8 ml DAM-TSC solution. The reaction mixture is heated at 100° for 15 min to develop the color and read at 520 nm. Standards of 100 μl (plus 100 μl of H2O) and 200 μl of 10−3 M urea were run with the samples.
Nitrite was assayed by Gieskes et al. (1991) adaptation of the method of Strickland and Parsons (1972) in which sulfanilamide in acid solution reacts with nitrite to form a diazo intermediate which is coupled to N-(1-naphthyl)-ethylenediamine (NEDA) to form a compound whose concentration is measured by absorbance at 543 nm. Two stock solutions were required: Acid-Sulfanilamide (AS) and NEDA. AS reagent was made adding 1 g of sulfanilamide to 10 ml concentrated HCl and 30 ml H2O and diluting to 50 ml with water. NEDA reagent was prepared by dissolving 0.5 g of NEDA in 50 ml water. About 10 to 100 μl of sample were brought to 2 ml with water and incubated with 100 μl of AS reagent. After 8 min, 100 μl of NEDA reagent was added and the mixture immediately mixed on a vortex mixer. The tubes were read after 10 min. Aliquots NaNO2 were run as standards.
Nitrate concentrations were measured by their UV absorbance at 220 nm by the method of Collos et al. (1999) with the diode array spectrophotometer as described above and corrected for the presence of nitrite. The assay is linear between 1 and 500 μM \({\text{NO}}_3^ - \).
HCN was derivitized according to the procedure of Chinaka et al. (2001). Stock solutions of 50 mM taurine and 2 mM 2,3-naphthalenedialdehyde were made in water. Sample volumes of 0.5 ml were mixed with 0.1 ml each of taurine and 2,3-naphthalenedialdehyde reagents and incubated for 10–30 min at room temperature to form a fluorescent derivative. 50 μl of the reaction mix was chromatographed on a Waters ODS-AQ 5 μm C18 reverse-phase HPLC column using a Shimadzu RF-535 Fluorescence Detector. Samples were eluted isocratically using 1:1 50 mM sodium acetate: methanol buffer at a flow rate of 1 ml/min. The excitation wavelength was 420 nm and the emission wavelength 460 nm.
Amino acids were determined by high performance liquid chromatography (HPLC) pre-column derivitization with o-phthalaldehyde (OPA) and N-acetyl-l-cysteine (NAC) according to the method of Zhao and Bada (1995). Fractions were injected onto a Phenomenex Luna C18 (2) 250 × 4.6 mm 100A 5 μm HPLC column and eluted with a 50 mM pH 5.5 sodium acetate–methanol gradient whose mobile phases consisted of buffer A, 50 mM pH 5.5 sodium acetate–methanol (92:8), and buffer B, 100% methanol, at a total flow rate of 1 ml/min. Fluorescence was monitored at 450 nm (excitation at 340) with a Shimadzu RF-535 Fluorescence Detector.
The HPLC column was equilibrated with buffer A at the beginning of a run and after 5 min the gradient was started to change the mobile phase to 63% buffer A at 15 min, then to 58% buffer A at 25 min and to 40% at 30 min. This concentration was held for 5 min and increased back to 100% A by 45 min. The column was then re-equilibrated with 100% buffer A for 10 min (to 55 min).
Results and Discussion
The spark discharge apparatus described in Schlesinger and Miller (1983) was used in our experiments. Water (100 ml) and 0.13 bar (100 mm) each of CO2 and N2 were subjected to the action of an electric discharge (generated by an Electrotechnics BD-50 Tesla coil) in a 3.1 l flask for 48 h at 23°C. Detectable amounts of HCN, NH3 (Table 1), along with urea, HCHO, CH3CHO, HOCCHO and OCHCO2H were produced, but only negligible yields of amino acids were obtained (Table 2 and Figs. 1 and 2). No visible insoluble organic polymer was observed, in contrast to the large amounts of polymeric material produced with reducing gas mixtures (Miller 1955). Amino acid yields were ∼10−2% based on input N2, similar to those reported previously (Schlesinger and Miller 1983).
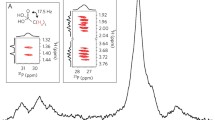
Typical HPLC chromatograms of the OPA-NAC derivitized amino acids detected in the various spark discharge experiments. Chromatograms labels: I, CO2/N2 not sparked (blank); II, CO2/N2, sparked with CaCO3, hydrolyzed without ascorbate; III, CO2/N2, sparked, hydrolyzed without ascorbate; IV, CO2/N2, sparked with CaCO3, hydrolyzed with ascorbate; V, amino acid standard. Amino acids: 1, DL aspartic acid; 2, DL glutamic acid; 3, DL serine; 4, glycine; 5, β-alanine; 6, DL alanine; 7, α-amino isobutyric acid; and 8, DL norleucine (internal standard). The D and L enantiomers of glutamic acid and serine are not separated under these chromatographic conditions
Yields of amino acids from spark discharge experiments (yields based on input nitrogen). Left 0.13 bar each of CO2 and N2 sparked for 48 h at room temperature. Right 0.13 bar each of CO2 and N2 sparked for 48 h at room temperature with 2 mmol CaCO3 added. For each amino acid, the right bar is the yield after hydrolysis with ascorbate added and the left bar is the yield without ascorbate. “Others” includes β-alanine, γ-aminobutyric acid, and α-aminoisobutyric acid
Electric discharges acting on CO2 + N2 gas mixtures over liquid water (Folsome et al. 1981) and on N2 + H2O (g; Zohner and Broda 1979; Wells 2000) mixtures produce large amounts of HNO2 and HNO3, along with lesser amounts of ammonia. This suggests that H2O vapor disproportionates to hydrogen and oxygen radicals which in turn act as both reducing and oxidizing agents. Thus N2 is likely partly converted to both ammonia and nitrite, which in turn is partially oxidized to nitrate (Zohner and Broda 1979; Summers and Khare 2007). Some of the CO2 is likely reduced to CO and CH4, although this was not directly measured. In the absence of a buffer the production of large amounts of nitrite and nitrate lowers the pH of the aqueous phase after sparking to 3.2 (see Table 1). Such low pH would inhibit the Strecker synthesis, which depends on the nucleophilicity of both ammonia and cyanide anion (both pKa’s ∼9.2), as well as the self-condensation of HCN, which proceeds optimally near its pKa (Ferris et al. 1978).
Due to the lowering of the pH during the reaction, the experiment was repeated but with solid CaCO3 as a buffer (200 mg, 2 mmol). This is several times greater than its solubility under these conditions, and most of this material remained undissolved during the course of the reaction. When the reaction was buffered with CaCO3, the pH of the aqueous phase after sparking was 7.1 (Table 1). Again, no visible organic polymer formation was observed, but the amino acid yields increased 2–15 fold (Table 2 and Figs. 1 and 2). Higher amino acid yields where observed when an oxidation inhibitor was added to the mixture prior to acid hydrolysis (Table 2 and Figs 1 and 2). This can be explained by the fact that typical procedures for analyzing amino acids from natural water samples largely result in their destruction due to the presence of nitrate/nitrite which oxidize the amino acids to nitrosoamines under acidic conditions (Robertson et al. 1987). When hydrolysis is carried out in the presence of ascorbic acid, which is known to inhibit oxidation (Robertson et al. 1987), the amount of recovered amino acids from seawater was increased a factor of 10–100 fold. A similar effect was observed when ascorbic acid was added to the spark discharge reaction mixtures prior to hydrolysis (Table 2 and Figs. 1 and 2).
To demonstrate that the amino acids found were not artifacts produced by contaminants in the reagents, a series of control experiments with each of the various reagents was carried out. No amino acids above background level were observed with any of the reagents. In addition we determined the level of CH4 and NH3 present in the starting CO2 and N2 and found CH4 to be present only in the CO2 used at a level of 48 ppm. This amount of CH4 could not produce amino acids comparable to the levels detected. To show that the source of the amino acids was not due to the reaction of the various nitrogen species produced in the reaction with ascorbic acid, we reacted ascorbate individually and in combination with ammonia, nitrite, and nitrate. Amino acid yields close to blank levels were produced in these reactions, indicating that the amino acids detected were produced from the electric discharge reaction.
The main amino acids produced in these experiments were serine, glutamic acid, glycine and alanine, along with traces of aspartic acid, α-aminoisobutyric acid, γ-aminobutyric acid and β-alanine (Figs. 1 and 2). The alanine was racemic within experimental error, demonstrating that it was not derived from contamination (aspartic acid is present in such trace amounts that small amounts of contamination likely yield a less than racemic ratio). Few amino acids are detected before acid hydrolysis, and the amounts of free cyanide, aldehydes and ammonia were low, suggesting that the intermediates may be bound as nitriles or other precursors.
We have also tested the effect of trace levels of O2 in the neutral gas mixture on the amino acid yields. It is generally held that amino acid synthesis is impossible in the presence of O2 (Kasting 1993a, b). To test this we exposed a mixture containing 2 mmol CaCO3, 100 ml H2O, 100 mm each CO2 and N2, and 10 mm O2 to the electric discharge for 48 h. To our surprise a small quantity of amino acids (Table 2), consisting almost entirely of glycine and racemic alanine, were still produced. Since the early Earth’s atmosphere was probably devoid of significant levels of oxygen until roughly 3 bya (Kasting and Catling 2003; Catling and Claire 2005), direct abiotic syntheses of compounds such as amino acids may have still taken place even after the origin of life and during the early rise of atmospheric O2.
Formation mechanisms and ocean chemistry. Low molecular weight hydrocarbons are formed from CH4 photopolymerization (Lasaga et al. 1971), and the self-condensation of HCN into polymers of undetermined structure is an efficient process which proceeds optimally near its pKa (Ferris et al. 1978). In contrast with previous reports (Miller 1955; Miller and Urey 1959; Folsome et al. 1981), we did not observe the synthesis of polymeric organic material in our experiments. The aqueous phase also remained clear when the reaction was buffered with CaCO3 and the pH remained close to neutral. The absence of insoluble polymeric material, combined with the observed low levels of free cyanide, suggests that most of the cyanide is rapidly converted to low molecular weight species such as amino nitriles.
Several mechanisms could account for the amino acids reported here. These include (1) the Strecker synthesis, in which hydrogen cyanide, ammonia and carbonyl compounds (aldehydes or ketones) react to form aminonitriles, which then undergo hydrolysis to form amino acids (Miller 1955); (2) the Bucherer–Bergs reaction which generates amino acids from hydantoins, themselves formed from cyanohydrins and ammonium carbonate or from HCN, ketones (or aldehydes) and ammonium carbonate (Bucherer and Lieb 1934; Taillades et al. 1998); and (3) formation from the hydrolysis of HCN oligomers (Oró and Kamat 1961; Ferris et al. 1978). The high yields of non-α-amino acids (Figs. 1 and 2) and the absence of visible HCN polymers suggest that none of these processes can completely account for the amino acids detected, and there may be other mechanisms operative as well. It is worth noting, however, that HCN oligomerization can occur below the threshold of visible observation.
In our analytical procedure, the reaction mixture was acid hydrolyzed in order to convert any precursors to amino acids. Under natural oceanic conditions with the pH near neutral, hydrolysis of the precursors would likely proceed slowly, but nevertheless take place over reasonably short geologic time scales. Acid hydrolysis simply expedites this hydrolysis process in the laboratory. As mentioned, the presence of nitrate and nitrite in the reaction mixture results in extensive oxidative decomposition of amino acids under acidic conditions and thus ascorbic acid was added to minimize this problem. The presence of carbonic, nitric and nitrous acids in the Earth’s primitive oceans may therefore have inhibited amino acid formation due to acidic conditions. Ascorbic acid is an unlikely prebiotic anti-oxidant. However, oxidation could have been inhibited by other available chemical species such as sulfides and reduced metal ions (Zohner and Broda 1979). We therefore assayed the ability of various potentially prebiotic oxidation inhibitors to protect against oxidation during hydrolytic work-up. We added 1.5 × 10−4 moles (∼100 fold molar excess over total nitrite + nitrate present in the solutions) of either FeCl2, Na2S, Na2SO3, FeSO4, pyrites, or sodium formate to 1 ml of discharge solution, which was dried under vacuum at room temperature, then HCl vapor-hydrolyzed and desalted. Only pyrites (10× over control recovery) and FeSO4 (2× over control recovery) were found to be able to protect against degradation to a significant degree using this molar ratio of reactants.
Although the composition of primitive seawater is uncertain, it has been suggested that (1) the pH was close to neutral (Morse and Mackenzie 1998); (2) the carbonate concentration was higher (Morse and Mackenzie 1998); (3) NaCl concentrations may have been twice present values (Morse and Mackenzie 1998); and (4) there were low but significant concentrations of Fe2+ and \({\text{SO}}_4^{2 - }\) (Zohner and Broda 1979; Walker and Brimblecombe 1985; Summers 1999). It seems reasonable that there was a significant excess of ferrous iron over nitrate/nitrite on the primitive Earth (Walker and Brimblecombe 1985) and in fact the reaction of nitrite with ferrous iron may have been an important source of ammonia in the primitive seas (Summers 1999).
Conclusions
Low-efficiency syntheses of amino acids have been reported under neutral atmospheric conditions (Abelson 1966; Folsome et al. 1981; Schlesinger and Miller 1983; Plankensteiner et al. 2006). The results presented here extend previous reports and demonstrate that neutral atmospheres can provide biochemical monomers in much higher yield than previously thought. While the Earth may have harbored a reducing atmosphere very early in its history, neutral conditions likely soon prevailed (Kasting and Catling 2003). We have shown here that contrary to previous findings, significant amounts of amino acids can be produced under these conditions. In our experiments the amounts of free cyanide, aldehydes and ammonia were low, suggesting that the intermediates may be bound as nitriles. The observed amino acids may be formed by several mechanisms, including the Strecker synthesis or, more likely, by the Bucherer–Bergs reaction. Although the amino acids observed and their relative abundances are similar to those found when oligomers formed by the self-condensation of HCN in aqueous solution are hydrolyzed (Ferris et al. 1978), the absence of visible polymeric organic material in our experiments may render this mechanism less likely in the simulations reported here.
The results presented here suggest that the low yields of amino acids previously reported may have been the result of oxidation of organic compounds during hydrolytic workup by nitrite and nitrate produced simultaneously in the reactions, as well as by the inhibition of synthesis caused by the low pH generated by the production of these species. Buffering the reaction solution with respect to pH and the addition of oxidation inhibitors such as ascorbic acid or Fe2+ prior to hydrolysis results in the recovery of up to several hundred times more amino acids than reported previously. Experiments with slightly reducing model atmospheres (Schlesinger and Miller 1983) suggest that the addition of traces of CH4 and/or H2 to neutral atmosphere simulations would further enhance the production of amino acids.
The amount of amino acids synthesized under neutral conditions reported here also suggests that estimates of the relative contributions of endogenous synthesis and extraterrestrial organic compounds to the primitive Earth (Chyba and Sagan 1992) may need to be reassessed. The reaction of precursor species such as NH3, HCN and HCHO derived from exogenous sources would be of less relevance if the overall pH of the oceans was low due to acidification from high fluxes of nitric and nitrous acids. Furthermore, if the early atmosphere were oxidizing or neutral and there was a significant flux of NO2 and NO3 from atmospheric reactions, extraterrestrial organics may also have been significantly oxidized in the absence of reduced species such as Fe2+ in the primitive oceans.
Using the estimates provided by Chyba and Sagan (1992) for the sources of prebiotic compounds on the primitive Earth, atmospheric synthesis in neutral atmospheres may have been competitive with exogenous delivery and other sources of organic compounds, provided the early oceans were buffered sufficiently with respect to pH and redox balance, although all of these sources may have contributed to the inventory of organic molecules.
As Mars’ atmosphere has likely been composed mainly of CO2 and N2 since soon after it was formed (Jakosky and Phillips 2001), the results presented here also have important implications for the synthesis of organic compounds during the early history of Mars.
References
Abelson PH (1966) Chemical events on the primitive earth. Proc Natl Acad Sci U S A 55:1365–1372
Annino JS, Giese RW (1976) Chapter 12, Nitrogenous compounds in clinical chemistry, principles and procedures, 4th edn. Little, Brown and Company, Boston, pp 157–160
Bada JL, Bigham C, Miller SL (1994) Impact melting of frozen oceans on the early Earth: implications for the origin of life. Proc Natl Acad Sci U S A 91:1248–1250
Bucherer HT, Lieb VA (1934) Über die Bildung substituierter Hydantoine aus Aldehyden und Ketonen. J Prakt Chem 141:5–43
Catling DC, Claire MW (2005) How Earth’s atmosphere evolved to an oxic state: a status report. Earth Planet Sci Lett 237:1–20
Chinaka S, Tanaka S, Takayama N, Tsuji N, Takou S, Ueda K (2001) High-sensitivity analysis of cyanide by capillary electrophoresis with fluorescence detection. Anal Sci 17:649–652
Chyba C, Sagan C (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355:125–132
Collos Y, Mornet F, Sciandra A, Waser N, Larson A, Harrison PJ (1999) An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J Appl Phycol 11:179–184
Evans RT (1968) Manual and automated methods for measuring urea based on a modification of its reaction with diacetyl monoxime and thiosemicarbazide. J Clin Path 21:527–532
Ferris JF, Joshi PC, Edelson EH, Lawless JG (1978) HCN: a plausible source of purines, pyrimidines and amino acids on the primitive Earth. J Mol Evol 11:293–311
Folsome C, Brittain A, Smith A, Chang S (1981) Hydrazines and carbohydrazides produced from oxidized carbon in Earth’s primitive environment. Nature 294:64–65
Gieskes JM, Gamo T, Brumsack H (1991) Chemical methods for interstitial water analysis aboard JOIDES resolution ocean drilling program, Texas A&M University Technical Note 15. p 48
Gough DO (1981) Solar interior structure and luminosity variations. Sol Phys 74:21–34
Haldane JBS (1928) The origin of life. Ration Annu 148:3–10
Holland HD (1962) Model for the evolution of the earth’s atmosphere. In: Engle EJ, James HL, Leonard BF (eds) Petrologic studies: a volume in honor of A. G. Buddington. Geological Society of America, Boulder, pp 447–477
Jakosky BM, Phillips RJ (2001) Mars’ volatile and climate history. Nature 412:237–244
Kasting JF (1993a) Earth’s early atmosphere. Science 259:920–926
Kasting JF (1993b) Early evolution of the atmosphere and ocean. In: Greenberg JM, Mendoza-Gomez CX, Pirronello V (eds) The chemistry of life’s origin. Kluwer Academic, Dordrecht, pp 149–176
Kasting JF, Catling D (2003) Evolution of a habitable planet. Ann Rev Astron Astrophys 41:429–463
Lasaga AC, Holland HD, Dwyer MJ (1971) Primordial oil slick. Science 174:53–55
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL (1955) Production of some organic compounds under possible primitive earth conditions. J Amer Chem Soc 77:2351–2361
Miller SL (1998) The endogenous synthesis of organic compounds. In: Brack A (ed) The molecular origins of life: assembling pieces of the puzzle. Cambridge University Press, Cambridge, pp 59–85
Miller SL, Urey HC (1959) Organic compound synthesis on the primitive Earth. Science 130:245–251
Miyakawa S, Yamanashi H, Kobayashi K, Cleaves HJ, Miller SL (2002) Prebiotic synthesis from CO atmospheres: Implications for the origins of life. Proc Natl Acad Sci U S A 99:14628–14631
Morse JW, Mackenzie FT (1998) Hadean ocean carbonate geochemistry. Aquat Geochem 4:301–319
Oró J, Kamat S (1961) Amino-acid synthesis from hydrogen cyanide under possible primitive Earth conditions. Nature 190:442–443
Plankensteiner K, Reiner H, Rode BM (2006) Amino acids on the rampant primordial Earth: electric discharges and the hot salty ocean. Mol Diver 10:3–7
Robertson K, Williams P, Bada JL (1987) Acid hydrolysis of dissolved combined amino acids in seawater: a precautionary note. Limnol Oceanogr 32:996–997
Schlesinger G, Miller SL (1983) Prebiotic synthesis in atmospheres containing CH4, CO and CO2. I. Amino acids. J Mol Evol 19:376–382
Sleep NH, Zahnle K (2001) Carbon dioxide cycling and implications for climate on ancient Earth. J Geophys Res 106:1373–1399
Solorzano L (1969) Determination of ammonia in natural waters by the phenol hypochlorite method. Limnol Oceanogr 14:799–801
Strickland JDH, Parsons TR (1972) II.7. Determination of reactive nitrite, in Bulletin 167. In: Strickland JDH, Parsons TR (eds) A practical handbook of seawater analysis. 2nd edn. Fisheries Research Board of Canada, Ottawa, pp 77–80
Summers DP (1999) Sources and sinks for ammonia and nitrite on the early Earth and the reaction of nitrite with ammonia. Orig Life Evol Biosph 29:33–46
Summers DP, Khare B (2007) Nitrogen fixation on early Mars and other terrestrial planets: experimental demonstration of abiotic fixation reactions to nitrite and nitrate. Astrobiology 7:333–341
Sutherland JD, Whitfield JN (1997) Prebiotic chemistry: a bioorganic perspective. Tetrahedron 53:11493–11527
Taillades J, Beuzelin I, Garrel L, Tabacik V, Bied C, Commeyras A (1998) N-carbamoyl-alpha-amino acids rather than free-alpha-amino acids formation in the primitive hydrosphere: a novel proposal for the emergence of prebiotic peptides. Orig Life Evol Biosph 28:61–77
Tian F, Toon O, Pavlov A, De Sterck H (2005) A hydrogen-rich early Earth atmosphere. Science 308:1014–1017
Walker JC, Brimblecombe P (1985) Iron and sulfur in the pre-biologic ocean. Precambrian Res 28:205–222
Wells J (2000) Icons of evolution. Regnery, Washington DC, p 338
Zhao M, Bada JL (1995) Determination of α-dialkylamino acids and their enantiomers in geological samples by high-performance liquid chromatography after derivitization with a chiral adduct of o-phthalaldehyde. J Chromatogr 690:55–63
Zohner A, Broda E (1979) Model experiments on nitrate and nitrate in simulated primeval conditions. Orig Life 9:291–298
Acknowledgments
This work was supported by the University of California Institute for Mexico and the USA (UC MEXUS) Program and the NASA Specialized Center of Research and Training in Exobiology. Stanley L. Miller passed away during the final preparation of the manuscript. We dedicate this work to his memory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Stanley L. Miller died May 20, 2007.
Rights and permissions
About this article
Cite this article
Cleaves, H.J., Chalmers, J.H., Lazcano, A. et al. A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres. Orig Life Evol Biosph 38, 105–115 (2008). https://doi.org/10.1007/s11084-007-9120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-007-9120-3