Abstract
Purpose
Optimal treatment for primary central nervous system lymphoma (PCNSL) comprises polychemotherapy induction with high-dose methotrexate followed by consolidation therapy, but there is no standard treatment regimen because of a lack of comparative trials examining efficacy or relative value. We performed a retrospective outcome and relative cost analysis on consolidation regimens to gain perspective on how cost and benefit can be weighed in medical decisions for patients with PCNSL.
Methods
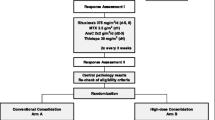
Patients with newly diagnosed PCNSL who completed consolidation at our institution from July 1, 2012, to March 1, 2019, were included. Patients completed etoposide/cytarabine (EA), high-dose cytarabine (HIDAC), or high-dose chemotherapy with autologous stem-cell rescue (HDC-ASCR) as consolidation regimen. Data were collected from the electronic medical record and our institution’s Value Driven Outcomes tool. Survival was analyzed as date of diagnosis to last known date of survival.
Results
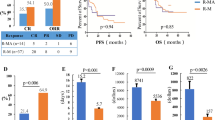
Of the 22 patients included in the study, 12 completed the EA regimen, 4 completed HDC-ASCR, and 6 completed HIDAC. Facility and pharmacy costs contributed most to the cost of each treatment. HDC-ASCR treatment was 50× the cost of the cheapest treatment, HIDAC. Outcomes were numerically superior with HDC-ASCR and HIDAC compared with EA (2-year progression-free survival 100% vs. 100% vs. 63.6%, respectively, p = 0.1915).
Conclusion
This small retrospective cost–benefit analysis provides evidence that HDC-ASCR may be a superior treatment for PCNSL but at a higher cost than other consolidation regimens. HIDAC may increase value for patients, including elderly patients, who are not appropriate candidates for HDC-ASCR when compared with EA.



Similar content being viewed by others
Data availability
The datasets generated during and or analyzed during the current study are not available.
References
Mendez JS, Ostrom QT, Gittleman H et al (2018) The elderly left behind-changes in survival trends of primary central nervous system Lymphoma over the past 4 decades. Neuro Oncol 20:687–694. https://doi.org/10.1093/neuonc/nox187
Grommes C, DeAngelis LM (2017) Primary CNS Lymphoma. J Clin Oncol 35:2410–2418. https://doi.org/10.1200/JCO.2017.72.7602
Rubenstein JL, Gupta NK, Mannis GN et al (2013) How I treat CNS Lymphomas. Blood 122:2318–2330. https://doi.org/10.1182/blood-2013-06-453084
Templates N (2014) Primary CNS Lymphoma rituximab primary CNS Lymphoma. J Clin Oncol 35:2013–2014
Abrey LE, Yahalom J, DeAngelis LM (2000) Treatment for primary CNS Lymphoma: the next step. J Clin Oncol 18:3144–3150. https://doi.org/10.1200/JCO.2000.18.17.3144
Omuro AMP, Ben-Porat LS, Panageas KS et al (2005) Delayed neurotoxicity in primary central nervous system Lymphoma. Arch Neurol. https://doi.org/10.1001/archneur.62.10.1595
Omuro A, Correa DD, DeAngelis LM et al (2015) R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS Lymphoma. Blood 125:1403–1410. https://doi.org/10.1182/blood-2014-10-604561
Rubenstein JL, Hsi ED, Johnson JL et al (2013) Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 31:3061–3068. https://doi.org/10.1200/JCO.2012.46.9957
Batchelor T, Giri S, Ruppert AS et al (2021) Myeloablative versus non-myeloablative consolidative chemotherapy for newly diagnosed primary central nervous system Lymphoma: results of CALGB 51101 (Alliance). J Clin Oncol 39:7506–7506. https://doi.org/10.1200/JCO.2021.39.15_suppl.7506
Schorb E, Finke J, Ferreri AJM et al (2016) High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS Lymphoma—a randomized phase III trial (MATRix). BMC Cancer 16:282. https://doi.org/10.1186/s12885-016-2311-4
Ferreri AJM, Cwynarski K, Pulczynski E et al (2017) Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal L. Lancet Haematol 4:e510–e523. https://doi.org/10.1016/S2352-3026(17)30174-6
Houillier C, Taillandier L, Dureau S et al (2019) Radiotherapy or autologous stem-cell transplantation for primary CNS Lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol 37:823–833. https://doi.org/10.1200/JCO.18.00306
Prica A, Chan K, Cheung M (2014) Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system Lymphoma: a cost-effectiveness analysis. Neuro Oncol 16:1384–1391. https://doi.org/10.1093/neuonc/nou057
Beca JM, Raza K, Mow E et al (2020) Cost-effectiveness analysis of rituximab with methotrexate, cytarabine and thiotepa for the treatment of patients with primary central nervous system Lymphoma. Leuk Lymphoma 61:1097–1107. https://doi.org/10.1080/10428194.2020.1711902
Kawamoto K, Martin CJ, Williams K et al (2015) Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc 22:223–235. https://doi.org/10.1136/amiajnl-2013-002511
Abrey LE, Ben-Porat L, Panageas KS et al (2006) Primary central nervous system Lymphoma: the memorial sloan-kettering cancer center prognostic model. J Clin Oncol 24:5711–5715. https://doi.org/10.1200/JCO.2006.08.2941
Schnipper LE, Davidson NE, Wollins DS et al (2016) Updating the american society of clinical oncology value framework: revisions and reflections in response to comments received. J Clin Oncol 34:2925–2934. https://doi.org/10.1200/JCO.2016.68.2518
Lee VS, Kawamoto K, Hess R et al (2016) Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA 316:1061. https://doi.org/10.1001/jama.2016.12226
Jafari L, Hussain J, Krishnadasan R et al (2019) Implementation of outpatient high-dose cytarabine (HiDAC) for AML: evaluation of the impact of transitioned outpatient chemotherapy in an oncology care model setting. Blood 134:2153–2153. https://doi.org/10.1182/blood-2019-132121
Schorb E, Fox CP, Fritsch K et al (2017) High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant 52:1113–1119. https://doi.org/10.1038/bmt.2017.23
Scordo M, Bhatt V, Hsu M et al (2017) A comprehensive assessment of toxicities in patients with central nervous system Lymphoma undergoing autologous stem cell transplantation using thiotepa, busulfan, and cyclophosphamide conditioning. Biol Blood Marrow Transpl 23:38–43. https://doi.org/10.1016/j.bbmt.2016.09.024
Creditor MC (1993) Hazards of hospitalization of the elderly. Ann Intern Med 118:219. https://doi.org/10.7326/0003-4819-118-3-199302010-00011
Funding
This work was supported by the Huntsman Cancer Institute Neurologic Disease Center, which is partially supported by the Huntsman Cancer Foundation. Use of the Research Informatics Shared Resource was provided by Huntsman Cancer Institute and supported by the Huntsman Cancer Institute’s Cancer Center Support Grant No. P30CA42014 from the National Cancer Institute of the National Institutes of Health. Resources of the Center for Neurologic Cancers were supported by Huntsman Cancer Institute and the Huntsman Cancer Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by SG and AM. Data analysis and interpretation was completed by SG, AM, JM, and AC. The first draft of the paper was written by SG and JM, and all authors commented on previous versions of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This is a retrospective observational study. The University of Utah IRB approved this study with a waiver of informed consent as protocol #66337.
Consent to participate
Exemption to informed consent was obtained through IRB approval for this retrospective study.
Consent to publish
Exemption to informed consent was obtained through IRB approval for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gelhard, S., Maxwell, A., Colman, H. et al. Consolidation regimens in primary central nervous system lymphoma: a single-center retrospective cohort evaluating survival outcomes and cost–benefit analysis. J Neurooncol 159, 293–300 (2022). https://doi.org/10.1007/s11060-022-04064-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04064-x