Abstract
Purpose
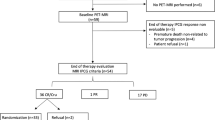
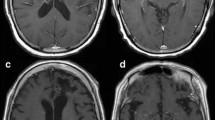
White matter changes (WMCs) can develop following systemic chemotherapy in patients with primary central nervous system lymphomas (PCNSLs), but the frequency and extent of these changes is not well characterized. This single center retrospective semi-quantitative study was performed to determine the rate, timing and grade of WMC on MRI in adult patients with newly-diagnosed radiotherapy-naïve PCNSL undergoing treatment with high-dose methotrexate (HD-MTX) with or without the addition of rituximab (-R).
Methods
Serial MRI scans of consecutive adult PCNSL patients treated with HD-MTX ± R were assessed for WMC comparing the pre-treatment to post-treatment scans utilizing a 0-to-8-point severity scoring system.
Results
Forty-seven PCNSL patients treated with either HD-MTX-R (n = 34; median age 66, 50% male) or HD-MTX (n = 13; median age 53, 54% male) were included in the analysis. WMC were detected in 62% (95% CI 46–76%) overall, in 68% of the HD-MTX-R, and in 46% of the HD-MTX group. Among patients with WMC (n = 29), WMC were first detected at an average of 2.8 months from beginning of therapy in the HD-MTX-R versus at 10.7 months in the HD-MTX group. Average WMC non-zero scores when first detected following the start of treatment were 2.5 (± 1.1) in HD-MTX-R and 1.5 (± 0.6) in HD-MTX.
Conclusions
Development of WMC in PCNSL patients treated with MTX and MTX-R is common. WMC changes appear to be more frequent, occur earlier and are more extensive in patients treated with HD-MTX-R compared to HD-MTX. Prospective studies are required to determine whether WMC correlate with survival or neurocognitive outcomes.


Similar content being viewed by others
Change history
22 November 2019
The following discrepancies and errors were found in the original publication:
References
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neurooncology 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, Grossman SA, Ye X (2014) High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 83:235–239. https://doi.org/10.1212/WNL.0000000000000593
Lai R, Abrey LE, Rosenblum MK, DeAngelis LM (2004) Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology 62:451–456
Salkade PR, Lim TA (2012) Methotrexate-induced acute toxic leukoencephalopathy. J Cancer Res Ther 8:292–296. https://doi.org/10.4103/0973-1482.98993
McKinney AM, Kieffer SA, Paylor RT, SantaCruz KS, Kendi A, Lucato L (2009) Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol 193:192–206. https://doi.org/10.2214/AJR.08.1176
Kaplan EL, Meier P (1958) Nonparametric-estimation from incomplete observations. J Am Stat Assoc 53:457–481. https://doi.org/10.2307/2281868
Brookmeyer R, Crowley J (1982) A confidence-interval for the median survival-time. Biometrics 38:29–41. https://doi.org/10.2307/2530286
Wilkinson HA, Fujiwara T, Rosenfeld S (1994) Synergistic effect between intraneoplastic methotrexate and radiation on experimental intracerebral rat gliosarcoma. Neurosurgery 34:665–668; discussion 668
Millot F, Dhondt JL, Mazingue F, Mechinaud F, Ingrand P, Guilhot F (1995) Changes of cerebral biopterin and biogenic amine metabolism in leukemic children receiving 5 g/m2 intravenous methotrexate. Pediatr Res 37:151–154. https://doi.org/10.1203/00006450-199502000-00004
Drachtman RA, Cole PD, Golden CB, James SJ, Melnyk S, Aisner J, Kamen BA (2002) Dextromethorphan is effective in the treatment of subacute methotrexate neurotoxicity. Pediatr Hematol Oncol 19:319–327. https://doi.org/10.1080/08880010290057336
Hyrien O, Dietrich J, Noble M (2010) Mathematical and experimental approaches to identify and predict the effects of chemotherapy on neuroglial precursors. Cancer Res 70:10051–10059. https://doi.org/10.1158/0008-5472.CAN-10-1400
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN et al (2019) Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176(1–2):43–55
Alderson L, Fetell MR, Sisti M, Hochberg F, Cohen M, Louis DN (1996) Sentinel lesions of primary CNS lymphoma. J Neurol Neurosurg Psychiatry 60:102–105. https://doi.org/10.1136/jnnp.60.1.102
Tan CS, Koralnik IJ (2010) Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 9(4):425–437
Trauninger A, Leel-Ossy E, Kamson DO, Poto L, Aradi M, Kover F, Imre M, Komaromy H, Erdelyi-Botor S, Patzko A, Pfund Z (2011) Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain 12:97–103. https://doi.org/10.1007/s10194-011-0299-3
Wiszniewska M, van Melle G, Devuyst G, Bogousslavsky J (2000) What is the significance of leukoaraiosis in patients with acute ischemic stroke. Stroke 31:2837–2837
Leys D, Englund E, Del Ser T, Inzitari D, Fazekas F, Bornstein N, Erkinjuntti T, Bowler JV, Pantoni L, Parnetti L, De Reuck J, Ferro J, Bogousslavsky J (1999) White matter changes in stroke patients—relationship with stroke subtype and outcome. Eur Neurol 42:67–75. https://doi.org/10.1159/000069414
Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R (1993) White-matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol Chic 50:818–824. https://doi.org/10.1001/archneur.1993.00540080029009
Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E (2000) Impact of white matter changes on clinical manifestation of Alzheimer’s disease—a quantitative study. Stroke 31:2182–2188. https://doi.org/10.1161/01.Str.31.9.2182
Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Moller HJ, Hampel H (2004) Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dement Geriatr Cogn 18:180–188. https://doi.org/10.1159/000079199
Fazekas F, Schmidt R, Scheltens P (1998) Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn 9:2–5. https://doi.org/10.1159/000051182
Acknowledgements
This study was supported by the Sidney Kimmel Comprehensive Cancer Center Core Grant P30CA006973 and the generosity of the Fernandes-Martins family and the Greening family.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest related to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Estephan, F., Ye, X., Dzaye, O. et al. White matter changes in primary central nervous system lymphoma patients treated with high-dose methotrexate with or without rituximab. J Neurooncol 145, 461–466 (2019). https://doi.org/10.1007/s11060-019-03279-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03279-9