Abstract
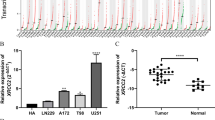
Cytochrome c oxidase subunit 7A2 (COX7A2) is a nuclear-encoded polypeptide involved in assembly and regulation of cytochrome c oxidase (COX). Changes in the respiratory chain as big complex are known to be associated with cancer, but little research has been performed to discover COX7A2 as a prognostic marker in glioma. In the present study, we investigated COX7A2 expression and its prognostic significance in glioma. Glioma surgical tissue samples were taken from 126 patients who had been followed up from 4 to 51 months. Immunohistochemistry were used to test COX7A2 expression in the 126 tumor samples. Eighty-six of 126 (68.3%) paraffin-embedded glioma biopsies showed high expression of COX7A2. Statistical analysis displayed that there was significant difference of COX7A2 expression level in patients categorized according to WHO classification. Kaplan–Meier survival analysis revealed that patients with higher COX7A2 expression had longer overall survival time and better prognosis. R2: microarray analysis based on Tumor Glioma French 284 database, Tumor Glioblastoma TCGA 540 database, and Tumor Glioma Kawaguchi 50 database testified that high expression of COX7A2 is associated with a good prognosis in patients with glioma. Multivariate analysis showed that COX7A2 high expression was an independent prognostic indicator for survival. Our results suggest that COX7A2 could be served as a valuable prognostic marker of glioma.



Similar content being viewed by others
References
Das S, Marsden PA (2013) Angiogenesis in glioblastoma. N Engl J Med 369:1561–1563. doi:10.1056/NEJMcibr1309402
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. doi:10.1056/NEJMoa1402121
Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, Shimamura T, Niida A, Motomura K, Ohka F, Yamamoto T, Tanahashi K, Ranjit M, Wakabayashi T, Yoshizato T, Kataoka K, Yoshida K, Nagata Y, Sato-Otsubo A, Tanaka H, Sanada M, Kondo Y, Nakamura H, Mizoguchi M, Abe T, Muragaki Y, Watanabe R, Ito I, Miyano S, Natsume A, Ogawa S (2015) Mutational landscape and clonalarchitecture in grade II and III gliomas. Nat Genet 47:458–468. doi:10.1038/ng.3273
Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D, Koelsche C, Bertoni A, Schweizer L, Korshunov A, Weiß EK, Schliesser MG, Radbruch A, Herold-Mende C, Roth P, Unterberg A, Hartmann C, Pietsch T, Reifenberger G, Lichter P, Radlwimmer B, Platten M, Pfister SM, von Deimling A, Weller M, Wick W (2014) Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol 128:561–571. doi:10.1007/s00401-014-1315-x
Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M, Felsberg J, Beyer U, Löffler-Wirth H, Kaulich K, Steinbach JP, Hartmann C, Gramatzki D, Schramm J, Westphal M, Schackert G, Simon M, Martens T, Boström J, Hagel C, Sabel M, Krex D, Tonn JC, Wick W, Noell S, Schlegel U, Radlwimmer B, Pietsch T, Loeffler M, von Deimling A, Binder H, Reifenberger G (2015) Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129:679–693. doi:10.1007/s00401-015-1409-0
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, Lopez M, Kalyanaraman B, Avadhani NG (2009) Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem J 420:439–449. doi:10.1042/BJ20090214
Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, Aebersold R, Auwerx J (2016) Systems proteomics of liver mitochondria function. Science 352:aad0189. doi:10.1126/science.aad0189
Wang L, McDonnell SK, Hebbring SJ, Cunningham JM, St Sauver J, Cerhan JR, Isaya G, Schaid DJ, Thibodeau SN (2008) Polymorphisms in mitochondrial genes and prostate cancer risk. Cancer Epidemiol Biomark Prev 17:3558–3566. doi:10.1158/1055-9965
Simonnet H, Alazard N, Pfeiffer K, Gallou C, Béroud C, Demont J, Bouvier R, Schägger H, Godinot C (2002) Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis 23:759–768
Oppenheimer SR, Mi D, Sanders ME, Caprioli RM (2010) Molecular analysis of tumor margins by MALDI mass spectrometry in renal carcinoma. J Proteome Res 9:2182–2190. doi:10.1021/pr900936z
Elsner M, Rauser S, Maier S, Schöne C, Balluff B, Meding S, Jung G, Nipp M, Sarioglu H, Maccarrone G, Aichler M, Feuchtinger A, Langer R, Jütting U, Feith M, Küster B, Ueffing M, Zitzelsberger H, Höfler H, Walch A (2012) MALDI imaging mass spectrometry reveals COX7A2, TAGLN2 and S100-A10 as novel prognostic markers in Barrett’s adenocarcinoma. J Proteomics 75:4693–4704. doi:10.1016/j.jprot.2012.02.012
Aichler M, Elsner M, Ludyga N, Feuchtinger A, Zangen V, Maier SK, Balluff B, Schöne C, Hierber L, Braselmann H, Meding S, Rauser S, Zischka H, Aubele M, Schmitt M, Feith M, Hauck SM, Ueffing M, Langer R, Kuster B, Zitzelsberger H, Höfler H, Walch AK (2013) Clinical response to chemotherapy in oesophageal adenocarcinoma patients is linked to defects in mitochondria. J Pathol 230:410–419. doi:10.1002/path.4199
Chrzanowska-Lightowlers ZM, Turnbull DM, Bindoff LA, Lightowlers RN (1993) An antisense oligodeoxynucleotide approach to investigate the function of the nuclear-encoded subunits of human cytochrome c oxidase. Biochem Biophys Res Commun 196:328–335
Wang HX, Zhao YX (2016) Prediction of genetic risk factors of atherosclerosis using various bioinformatic tools. Genet Mol Res 15:gmr7347. doi:10.4238/gmr.15027347
Lin CH, Liao CC, Huang CH, Tung YT, Chang HC, Hsu MC, Huang CC (2017) Proteomics analysis to identify and characterize the biomarkers and physical activities of non-frail and frail older adults. Int J Med Sci 14:231–239. doi:10.7150/ijms.17627
Singh KK (2004) Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann N Y Acad Sci 1019:260–264. doi:10.1196/annals.1297.043
Johnson RF, Perkins ND (2012) Nuclear factor-kappaB, p53, and mitochondria: regulation of cellular metabolism and the Warburg effect. Trends Biochem Sci 37:317–324. doi:10.1016/j.tibs.2012.04.002
Lopci E, Riva M, Olivari L, Raneri F, Soffietti R, Piccardo A, Bizzi A, Navarria P, Ascolese AM, Rudà R, Fernandes B, Pessina F, Grimaldi M, Simonelli M, Rossi M, Alfieri T, Zucali PA, Scorsetti M, Bello L, Chiti A (2017) Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging 44:1155–1164. doi:10.1007/s00259-017-3618-3
Babu R, Komisarow JM, Agarwal VJ, Rahimpour S, Iyer A, Britt D, Karikari IO, Grossi PM, Thomas S, Friedman AH, Adamson C (2016) Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg 124:998–1007. doi:10.3171/2015.4.JNS142200
Theeler BJ, Yung WK, Fuller GN, De Groot JF (2012) Moving toward molecular classification of diffuse gliomas in adults. Neurology 79:1917–1926. doi:10.1212/WNL.0b013e318271f7cb
Chen D, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N (2017) The oxido-metabolic driver ATF4 enhances temozolamide chemo-resistance in human gliomas. Oncotarget 8:51164–51176. doi:10.18632/oncotarget.17737
Wang HH, Chang TY, Lin WC, Wei K3, Shin JW (2017) GADD45A plays a protective role against temozolomide treatment in glioblastoma cells. Sci Rep 7:8814. doi:10.1038/s41598-017-06851-3
Maciaczyk D, Picard D, Zhao L, Koch K, Herrera-Rios D, Li G, Marquardt V, Pauck D, Hoerbelt T, Zhang W, Ouwens DM, Remke M, Jiang T, Steiger HJ, Maciaczyk J, Kahlert UD (2017) CBF1 is clinically prognostic and serves as a target to block cellular invasion and chemoresistance of EMT-like glioblastoma cells. Br J Cancer 117:102–112. doi:10.1038/bjc.2017.157
Acknowledgements
This work was supported by National Natural Science Foundation of China (81372692, 81472315, 81773290), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2014BAI04B01), Science and Technology Program of Guangdong (2016A020213006), Natural Science Foundation of Guangdong Province (2014A030313167, 2017A030313497).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the Ethics Committee of Southern Medical University and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, S., Li, Y., Yi, G. et al. Overexpression of COX7A2 is associated with a good prognosis in patients with glioma. J Neurooncol 136, 41–50 (2018). https://doi.org/10.1007/s11060-017-2637-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2637-z