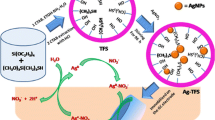
Abstract
Reducing AgNO\(_3\) by glucose at basic pH coated the surface of silica spheres were coated with a high density of hemispherical silver nanoparticles by direct reduction. The average diameter of the nanoparticles was 3.2 \(\pm \) 1 nm. A much lower silver concentration than is a standard favored heterogeneous nucleation of silver on the silica surface at the expense of homogeneous nucleation in solution. The slow growth rate of the nuclei promoted the formation of discrete silver particles rather than a continuous shell. Based on scanning electron microscopy and transmission electron microscopy, the surface coverage of silver seed particles was as high as 25 % at 10 °C without prior functionalization of the silica. The particles were composed of metallic silver based on X-ray photoelectron spectroscopy. There was a sharp increase in the silver surface coverage and a decrease in the particle size when the temperature was raised from 5 to 10 °C and the amount of silica was decreased from 0.2 to 0.025 V/V%. The size was controlled by the diffusion barrier through the ion shell surrounding the silica spheres and by maintaining reaction conditions where the particles on the surface compete for silver.
Graphical abstract






Similar content being viewed by others
References
Alvarez-Puebla RA, Arceo E, Goulet PJG, Garrido JJ, Aroca RF (2005) Role of nanoparticle surface charge in surface-enhanced Raman scattering. J Phys Chem B 109(9):3787–3792. doi:10.1021/jp045015o
Bao X, Muhler M, Schedel-Niedrig Th, Schlögl R (1996) Interaction of oxygen with silver at high temperature and atmospheric pressure: a spectroscopic and structural analysis of a strongly bound surface species. Phys Rev B 54:2249–2262. doi:10.1103/PhysRevB.54.2249
Bogush GH, Tracy MA, Zukoski CF IV (1988) Preparation of monodisperse silica particles: control of size and mass fraction. J Non Cryst Solids 104(1):95–106. doi:10.1016/0022-3093(88)90187-1
Brinson BE, Lassiter JB, Levin CS, Bardhan R, Mirin N, Halas NJ (2008) Nanoshells made easy: improving Au layer growth on nanoparticle surfaces. Langmuir 24(24):14166–14171. doi:10.1021/la802049p
Brito-Silva AM, Sobral-Filho RG, Barbosa-Silva R, de Araújo CB, Galembeck A, Brolo AG (2013) Improved synthesis of gold and silver nanoshells. Langmuir 29(13):4366–4372. doi:10.1021/la3050626
Choma J, Dziura A, Jamiłoa D, Nyga P, Jaroniec M (2011) Preparation and properties of silica-gold core-shell particles. Colloids Surf A 373(1–3):167–171. doi:10.1016/j.colsurfa.2010.10.046
Choma J, Jamioła D, Ludwinowicz J, Jaroniec M (2012) Deposition of silver nanoparticles on silica spheres and rods. Colloids Surf A 411:74–79. doi:10.1016/j.colsurfa.2012.07.004
Dementeva OV, Filippenko MA, Groman KE, Rudoy VM (2012) New multifunctional nanoparticles with mesoporous cores and silver shells. Colloid J 74(4):440–444. doi:10.1134/S1061933X12040059
Goodwin JW, Harbron RS, Reynolds PA (1990) Functionalization of colloidal silica and silica surfaces via silylation reactions. Colloid Polym Sci 268(8):766–777. doi:10.1007/BF01411109
Heuck FCA, Staufer U (2011) Electroless deposition and structuring of silver electrodes in closed microfluidic capillaries. J Microelectromech Syst 20(2):451–459. doi:10.1109/JMEMS.2011.2105254
Homan K, Shah J, Gomez S, Gensler H, Karpiouk A, Brannon-Peppas L, Emelianov S (2010) Silver nanosystems for photoacoustic imaging and image-guided therapy. J Biomed Opt 15(2):021316. doi:10.1117/1.3365937
Jackson JB, Halas NJ (2001) Silver nanoshells: variations in morphologies and optical properties. J Phys Chem B 105(14):2743–2746. doi:10.1021/jp003868k
Jiang Z, Liu C (2003) Seed-mediated growth technique for the preparation of a silver nanoshell on a silica sphere. J Phys Chem B 107(45):12411–12415. doi:10.1021/jp035060g
Kedziora A, Strek W, Kepinski L, Bugla-Ploskonska G, Doroszkiewicz W (2012) Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J Sol Gel Sci Technol 62(1):79–86. doi:10.1007/s10971-012-2688-8
Kelly MA (2003) Analyzing insulators with XPS and AES. In: Briggs D, Grant JT (eds) Surface analysis by auger and X-ray photoelectron spectroscopy. IMPublications, Charlton, pp 191–210
Kim J, Bryan WW, Randall Lee T (2008) Preparation, characterization, and optical properties of gold, silver, and gold-silver alloy nanoshells having silica cores. Langmuir 24(19):11147–11152. doi:10.1021/la8016497
Kobayashi Y, Salgueiriño-Maceira V, Liz-Marzán LM (2001) Deposition of silver nanoparticles on silica spheres by pretreatment steps in electroless plating. Chem Mater 13(5):1630–1633. doi:10.1021/cm001240g
Lee J, Kim D, Jun Y, Oh S (2006) Preparation of silica-silver heterogeneous nanocomposite particles by one-pot preparation strategy using polyol process: Size-controlled immobilization of silver nanoparticles. Mater Res Bull 41(8):1407–1416. doi:10.1016/j.materresbull.2006.02.010
Lopez N, Janssens TVW, Clausen BS, Xu Y, Mavrikakis M, Bligaard T, Nørskov JK (2004) On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. J Catal 223(1):232–235. doi:10.1016/j.jcat.2004.01.001
Markova Z, Siskova K, Filip J, Safarova K, Prucek R, Panacek A, Kolar M, Zboril R (2012) Chitosan-based synthesis of magnetically-driven nanocomposites with biogenic magnetite core, controlled silver size, and high antimicrobial activity. Green Chem 14:2550–2558. doi:10.1039/C2GC35545K
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1995) Handbook of X-ray photoelectron spectroscopy. Physical Electronics Inc., Eden Prairie
Pan K, Liang Y, Pu Y, Hsu Y, Yeh J, Shih HC (2014) Studies on the photocatalysis of core-shelled Si\({\rm O}_2\)-Ag nanospheres by controlled surface plasmon resonance under visible light. Appl Surf Sci 311:399–404. doi:10.1016/j.apsusc.2014.05.074
Park S, Park M, Han P, Lee S (2006) The effect of pH-adjusted gold colloids on the formation of gold clusters over APTMS-coated silica cores. Bull Korean Chem Soc 27:1341–1345. doi:10.5012/bkcs.2006.27.9.1341
Peterson MSM, Bouwman J, Chen A, Deutsch M (2007) Inorganic metallodielectric materials fabricated using two single-step methods based on the Tollens process. J Colloid Interface Sci 306(1):41–49. doi:10.1016/j.jcis.2006.10.013
Preston TC, Signorell R (2009) Growth and optical properties of gold nanoshells prior to the formation of a continuous metallic layer. ACS Nano 3(11):3696–3706. doi:10.1021/nn900883d
Rempel JY, Bawendi MG, Jensen KF (2009) Insights into the kinetics of semiconductor nanocrystal nucleation and growth. J Am Chem Soc 131(12):4479–4489. doi:10.1021/ja809156t
Senapati S, Srivastava SK, Singh SB, Kulkarni AR (2014) SERS active Ag encapsulated Fe@Si\({\rm O}_2\) nanorods in electromagnetic wave absorption and crystal violet detection. Environ Res 135:95–104. doi:10.1016/j.envres.2014.08.026
Sohn Y (2013) Si\({\rm O}_2\) nanospheres modified by Ag nanoparticles: surface charging and CO oxidation activity. J Mol Catal A 379:59–67. doi:10.1016/j.molcata.2013.07.015
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69. doi:10.1016/0021-9797(68)90272-5
Tzounis L, Contreras-Caceres R, Schellkopf L, Jehnichen D, Fischer D, Cai C, Uhlmann P, Stamm M (2014) Controlled growth of Ag nanoparticles decorated onto the surface of Si\({\rm O}_2\) spheres: a nanohybrid system with combined SERS and catalytic properties. RSC Adv 4:17846–17855. doi:10.1039/C4RA00121D
Waterhouse GIN, Bowmaker GA, Metson JB (2004) Mechanism and active sites for the partial oxidation of methanol to formaldehyde over an electrolytic silver catalyst. Appl Catal A Gen 265(1):85–101. doi:10.1016/j.apcata.2004.01.016
Zhang J, Liu H, Wang Z, Ming N (2007) Preparation and optical properties of silica@Ag-Cu alloy core-shell composite colloids. J Solid State Chem 180(4):1291–1297. doi:10.1016/j.jssc.2007.01.035
Zhang J, Liu J, Wang S, Zhan P, Wang Z, Ming N (2004) Facile methods to coat polystyrene and flica colloids with metal. Adv Funct Mater 14(11):1089–1096. doi:10.1002/adfm.200400119
Zhang S, Ren F, Wu W, Zhou J, Sun L, Xiao X, Jiang C (2012) Modified in situ and self-catalytic growth method for fabrication of Ag-coated nanocomposites with tailorable optical properties. J Nanopart Res 14(9):1–13. doi:10.1007/s11051-012-1105-0
Acknowledgments
The authors thank Pablo Mancheno for his help with collection and analysis of XPS data. The authors also thank Lance Hubbard for his assistance collecting SEM images and for useful conversations about electrostatics in colloidal systems.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purdy, S.C., Muscat, A.J. Coating nonfunctionalized silica spheres with a high density of discrete silver nanoparticles. J Nanopart Res 18, 70 (2016). https://doi.org/10.1007/s11051-016-3371-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3371-8