Abstract
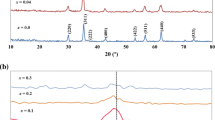
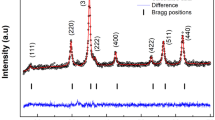
ZnFe2O4 bulk material shows a normal-spinel structure and a closely defined composition at Zn2+/Fe3+ ≅ 0.5. However, the composition of zinc ferrite, prepared as nanoparticles, can be varied in a broad range without losing the single-phase spinel structure. In this article, structural mechanisms enabling this non-stoichiometry were studied using the X-ray absorption fine structure (EXAFS) in combination with X-ray diffractometry (XRD), transmission electron microscopy (TEM), and magnetic measurements. Nanoparticles with a narrow size distribution were synthesized using co-precipitation in water-in-oil microemulsions. First, the structure of the stoichiometric zinc-ferrite nanoparticles was studied in dependence of their size and the annealing temperature. EXAFS analysis showed that the degree of inversion x (as defined in the compound formula (Zn1 − x Fe x )[Fe2 − x Zn x ]O4, with round and square brackets representing the tetrahedral and octahedral sites, respectively) increased with decreasing nanoparticles size. The structure of the stoichiometric nanoparticles and the nanoparticles of comparable size displaying Zn/Fe ratio of 0.2 (Fe-rich) and 0.7 (Zn-rich) were then compared. Analysis showed that the non-stoichiometry is structurally compensated predominantly in the core of the nanoparticle by the adjusted distribution of Zn and Fe ions over the two sublattices of the spinel structure.





Similar content being viewed by others
References
Ammar S, Jouini N, Fievet F, Stephan O, Marhic C, Richard M, Villain F, Chartier dit Moulin Ch, Brice S, Sainctavit Ph (2004) Influence of the synthesis parameters on the cation distribution of ZnFe2O4 nanoparticles obtained by forced hydrolysis in polyol medium. J Non Cryst Solids 345 & 346:658–662
Brockhouse BN, Corliss LM, Hastings JM (1955) Multiple scattering of slow neutrons by flat specimen and magnetic scattering by zinc ferrite. Phys Rev 98:1721–1727
Calvin S, Carpenter EE, Ravel B, Harris VG, Morrison SA (2002) Multiedge refinement of extended x-ray absorption fine structure of manganese zinc ferrite nanoparticles. Phys Rev B66:224405-1–224405-13
Hamdeh HH, Ho JC, Oliver SA, Willey RJ, Oliveri G, Busca GJ (1997) Magnetic properties of partially-inverted zinc ferrite aerogel powders. Appl Phys 81:1851–1857
Jeyadevan B, Tohji T, Nakatsuka KJ (1994) Structure-analysis of coprecipitated ZnFe2O4 by extended X-ray absorption fine-structure. Appl Phys 76:6325–6327
Kamiyama T, Haneda K, Sato T, Ikeda S, Asano H (1992) Cation distribution in ZnFe2O4 fine particles studied by neutron powder diffraction. Solid State Commun 81:563–566
Koenig U, Chol G (1968) Roentgenbeugungs- und Neutronenbeugungsuntersuchungen an Ferriten der Reihe MnxZn1−XFe2O4. J Appl Cryst 1:124–126
Ligenza S (1976) Study of spin interaction in manganese-substituted zinc ferrite by neutron spectroscopy. Phys Stat Solidi (B) 75:301–310
Lotgering FK (1966) The influence of Fe3+ ions at tetrahedral sites on the magnetic properties of ZnFe2O4. J Phys Chem Solids 27:139–145
Lykasov AA, D’yachuk VV, Pavlovskaya MS (1991) Univariant equilibria in Fe–Zn–O system. Neorg Mater 27:2153–2157; Inorg Mater 27:446–449
Makovec D, Drofenik M (2008) Non-stoichiometric zinc-ferrite spinel nanoparticles. J Nanopart Res 10:131–141
Mason B (1947) Mineralogical aspects of the system Fe3O4–Mn3O4–ZnMn2O4–ZnFe2O4. Am Miner 32:426–432
O’Neill HS (1992) Temperature-dependence of the cation distribution in zinc ferrite (ZnFe2O4) from powder XRD structural refinement. Eur J Miner 4:571–580
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Rehr JJ, Albers RC, Zabinsky SI (1992) High-order multiple-scattering calculations of X-ray-absorption fine-structure. Phys Rev Lett 69:3397–3400
Sato T, Haneda K, Seki M, Iijima T (1990) Morphology and magnetic properties of ultrafine ZnFe2O4 particles. Appl Phys A50:13–16
Smit J, Wijn HPJ (1959) Ferrites. Philips’ Technical Library, Eindhoven, The Netherlands
Tanida K, Kitamura T (1984) System Fe2O3–ZnO: subsolidus relations in air. Tohoku Daigaku Senko Seiren Kenkyusho Iho 40:71–76
Acknowledgments
This study was supported by the Slovenian Research Agency, the Ministry of Higher Education, Science and Technology of the Republic of Slovenia within the National Research Program, and by DESY and the European Community under Contract RII3-CT-2004-506008 (IA-SFS). Provision of synchrotron radiation facilities by HASYLAB (project II-04-065 EC) is acknowledged. The authors would also like to thank Sašo Gyergyek for help with the magnetic measurements and E. Welter of HASYLAB for expert advice on beamline operation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Makovec, D., Kodre, A., Arčon, I. et al. The structure of compositionally constrained zinc-ferrite spinel nanoparticles. J Nanopart Res 13, 1781–1790 (2011). https://doi.org/10.1007/s11051-010-9929-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-9929-y