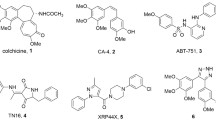
Abstract
A representative series of structural analogs of the antimitotic tripeptides hemiasterlins have been designed and synthesized, as potential inhibitors of tubulin polymerization. Relying also on a computational approach, we aimed to explore unknown extensive changes at the C-fragment, by incorporating the conformationally required double bond into five- and six-membered rings. Key steps of the synthetic strategy are a dynamic resolution affording the A-fragment in 97 % ee and the preparation of six new cyclic C fragments, all potentially able to interact with tubulin by means of H bonds. Unexpectedly, biological evaluation of these analogs did not provide evidences neither for cytotoxic effect nor for inhibition of tubulin polymerization.








Similar content being viewed by others
Notes
Scores are reported as obtained by the similarity analysis function implemented in the Spartan’06 software. The score is defined as [\((1-R2)/N\)], where R2 is the rms distance between template and molecule centers, and N is the number of similarity centers.
References
Kingston DGI (2009) Tubulin-interactive natural products as anticancer agents. J Nat Prod 72:507–515. doi:10.1021/np800568j
Beckers T, Mahboobi S (2003) Natural, semisynthetic and synthetic microtubule inhibitors for cancer therapy. Drugs Fut 28:767. doi:10.1358/dof.2003.028.08.744356
Jordan A, Hadfield JA, Lawrence NJ, McGown AT (1998) Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev 18:259–296. doi:10.1002/(SICI)1098-1128(199807)18:4<259:AID-MED3>3.0.CO;2-U
Ganguly A, Cabral F (2011) New insights into mechanisms of resistance to microtubule inhibitors. Biochim Biophys Acta 1816:164–171. doi:10.1016/j.bbcan.2011.06.001
Mania S, Macapinlac M, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, Colevas D (2004) The clinical development of new mitotic inhibitors that stabilize the microtubule. Anti-Cancer Drugs 15:553–558. doi:10.1097/01.cad.0000131681.21637.b2
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803. doi:10.1038/nrd3253
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2009) Drug development from marine natural products. Nat Rev Drug Discov 8:69–85. doi:10.1038/nrd2487
Coleman JE, de Silva ED, Fangming K, Andersen RJ, Allen TM (1995) Cytotoxic peptides from the marine sponge Cymbastela sp. Tetrahedron 51:10653–10662. doi:10.1016/0040-4020(95)00646-P
Gamble WR, Durso NA, Fuller RW, Westergaard CK, Johnson TR, Sackett DL, Hamel E, Cardellina JH 2nd, Boyd MR (1999) Cytotoxic and tubulin-interactive hemiasterlins from Auletta sp. and Siphonochalina spp. sponges. Bioorg Med Chem 7:1611–1615. doi:10.1016/S0968-0896(99)00089-9
Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M (1994) Hemiasterlin and geodiamolide TA; two new cytotoxic peptides from the marine sponge Hemiasterella minor (Kirkpatrick). Tetrahedron Lett 35:4453–4456. doi:10.1016/S0040-4039(00)73382-X
Anderson HJ, Coleman JE, Andersen RJ, Roberge M (1997) Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother Pharmacol 39:223–226. doi:10.1007/s002800050564.ISSN:0344-5704
Bai R, Durso NA, Sackett DL, Hamel E (1999) Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulin: comparison with dolastatin 10 and cryptophycin 1. Biochemistry 38:14302–14310. doi:10.1021/bi991323e
Vedejs E, Kongkittingam C (2001) A total synthesis of (-)-hemiasterlin using N-Bts methodology. J Org Chem 66:7355–7364. doi:10.1021/jo0104882
Kuznetsov G, TenDyke K, Towle MJ, Cheng H, Liu J, Marsh JP, Schiller SER, Spyvee MR, Yang H, Seletsky BM, Shaffer CJ, Marceau V, Yao Y, Suh EM, Campagna S, Fang FG, Kowalczyk JJ, Littlefield BA (2009) Tubulin-based antimitotic mechanism of E7974, a novel analogue of the marine sponge natural product hemiasterlin. Mol Cancer Ther 8:2852–2860. doi:10.1158/1535-7163.MCT-09-0301
Zask A, Birnberg G, Cheung K, Kaplan J, Niu C, Norton E, Suayan R, Yamashita A, Cole D, Tang Z, Krishnamurthy G, Williamson R, Khafizova G, Musto S, Hernandez R, Annable T, Yang X, Discafani C, Beyer C, Greenberger LM, Loganzo F, Ayral-Kaloustian S (2004) Synthesis and biological activity of analogues of the antimicrotubule agent N,\(\beta , \beta \)-Trimethyl-l-phenylalanyl-N1-[(1S,2E)-3-carboxy-1-isopropylbut-2-enyl]- N1,3-dimethyl-l-valinamide (HTI-286). J Med Chem 47:4774–4786. doi:10.1021/jm040056u
Niu C, Ho DM, Zask A, Ayral-Kaloustian S (2010) Absolute configurations of tubulin inhibitors taltobulin (HTI-286) and HTI-042 characterized by X-ray diffraction analysis and NMR studies. Bioorg Med Chem Lett 20:1535–1538. doi:10.1016/j.bmcl.2010.01.047
Zask A, Kaplan J, Musto S, Loganzo F (2005) Hybrids of the hemiasterlin analogue taltobulin and the dolastatins are potent antimicrotubule agents. J Am Chem Soc 127:17667–17671. doi:10.1021/ja053663v
Hadaschik BA, Adomat H, Fazli L, Fradet Y, Andersen RJ, Gleave ME, So A (2008) Intravesical chemotherapy of high-grade bladder cancer with HTI-286, a synthetic analogue of the marine sponge product hemiasterlin. Clin Cancer Res 14:1510–1518. doi:10.1158/1078-0432.CCR-07-4475
Hadaschik BA, Ettinger S, Sowery RD, Zoubeidi A, Andersen RJ, Roberge M, Gleave ME (2008) Targeting prostate cancer with HTI-286, a synthetic analog of the marine sponge product hemiasterlin. Int J Cancer 122:2368–2376. doi:10.1002/ijc.23406
Ayral-Kaloustian S, Zask A (2005) Taltobulin. Drugs Fut 30:254. doi:10.1358/dof.2005.030.03.886482
Loganzo F, Hari M, Annable T, Tan X, Morilla DB, Musto S, Zask A, Kaplan J, Minnick AAJr, May MK, Ayral-Kaloustian S, Poruchynsky MS, Fojo T, Greenberger LM (2004) Cells resistant to HTI-286 do not overexpress P-glycoprotein but have reduced drug accumulation and a point mutation in \(\alpha \)-tubulin. Mol Cancer Ther 3:1319–1327
Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, Tan X, Hardy C, Hernandez R, Baxter M, Singanallore T, Khafizova G, Poruchynsky MS, Fojo T, Nieman JA, Ayral-Kaloustian S, Zask A, Andersen RJ, Greenberger LM (2003) HTI-286, A synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res 63:1838–1845
Nieman JA, Coleman JE, Wallace DJ, Piers E, Lim LY, Roberge M, Andersen RJ (2003) Synthesis and antimitotic/cytotoxic activity of hemiasterlin analogues. J Nat Prod 66:183–199. doi:10.1021/np020375t
Andersen RJ, Coleman JE, Piers E, Wallace DJ (1997) Total synthesis of (-)-hemiasterlin, a structurally novel tripeptide that exhibits potent cytotoxic activity. Tetrahedron Lett 38:317–320. doi:10.1016/S0040-4039(96)02335-0
Hsu L-C, Durrant DE, Huang C-C, Chi N-W, Baruchello R, Rondanin R, Rullo C, Marchetti P, Grisolia G, Simoni D, Lee RM (2012) Development of hemiasterlin derivatives as potential anticancer agents that inhibit tubulin polymerization and synergize with a stilbene tubulin inhibitor. Invest New Drugs 30:1379–1388. doi:10.1007/s10637-011-9702-9
Simoni D, Lee RM, Durrant DE, Chi N-W, Baruchello R, Rondanin R, Rullo C, Marchetti P (2010) Versatile synthesis of new cytotoxic agents structurally related to hemiasterlins. Bioorg Med Chem Lett 20:3431–3435. doi:10.1016/j.bmcl.2010.03.098
Qie J, Zhou W, Zhao X, He J, Zhang Y, Liu K (2009) Hemiasterlin analogues with unnatural amino acids at the N-terminal and their inhibitory activity on tumor cells. Int J Pept Res Ther 15:187–194. doi:10.1007/s10989-009-9168-1
Yamashita A, Norton EB, Kaplan JA, Niu C, Loganzo F, Hernandez R, Beyer CF, Annable T, Musto S, Discafani C, Zask A, Ayral-Kaloustian S (2004) Synthesis and activity of novel analogs of hemiasterlin as inhibitors of tubulin polymerization: modification of the A segment. Bioorg Med Chem Lett 14:5317–5322. doi:10.1016/j.bmcl.2004.08.024
Zask A, Birnberg G, Cheung K, Kaplan J, Niu C, Norton E, Yamashita A, Beyer C, Krishnamurthy G, Greenberger LM, Loganzo F, Ayral-Kaloustian S (2004) D-Piece modifications of the hemiasterlin analog HTI-286 produce potent tubulin inhibitors. Bioorg Med Chem Lett 14:4353–4358. doi:10.1016/j.bmcl.2004.05.005
Milton MJ, Thomas Williamson R (2006) Mapping the bound conformation and protein interactions of microtubule destabilizing peptides by STD-NMR spectroscopy. Bioorg Med Chem Lett 16:4279–4282. doi:10.1016/j.bmcl.2006.05.067
Ravi M, Zask A, Rush TS (2005) Structure-based identification of the binding site for the hemiasterlin analogue HTI-286 on tubulin. Biochemistry 44:15871–15879. doi:10.1021/bi051268b
Mitra A, Sept D (2004) Localization of the antimitotic peptide and depsipeptide binding site on \(\beta \)-tubulin. Biochemistry 43:13955–13962. doi:10.1021/bi0487387
Spartan’08, Wavefunction Inc, Irvine, CA; Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O’Neill DP, DiStasio RA Jr, Lochan RC, Wang T, Beran, GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khalliulin RZ, Klunzinger P, Lee AM, Lee MS, Liang WZ, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee YM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock III HL, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF, Kong J, Krylov AI, Gill PMW, Head-Gordon M (2006) Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys 8:3172–3191. doi:10.1039/B517914A
Mitchell D, Hay LA, Koenig TM, McDaniel S, Nissen JS, Audia JE (2005) Classical and dynamic resolution of 1-amino-3-methyl-1,3,4,5-tetrahydrobenzo[d]azepin-2-one. Tetrahedron: Asymmetry 16:3814–3819. doi:10.1016/j.tetasy.2005.10.037
Lim HJ, Gallucci JC, RajanBabu TV (2010) Annulated diketopiperazines from dipeptides or Schöllkopf reagents via tandem cyclization-intramolecular N-arylation. Org Lett 12:2162–2165. doi:10.1021/ol100663
Malkov AV, Vranková K, Černý M, Kočovský P (2009) On the selective N-methylation of BOC-protected amino acids. J Org Chem 74:8425–8427. doi:10.1021/jo9016293
Mangette JE, Johnson MR, Le V-D, Shenoy RA, Roark H, Stier M, Belliotti T, Capiris T, Guzzo PR (2009) The preparation of optically active \(\alpha \)-amino 4H-[1,2,4]oxadiazol-5-ones from optically active \(\alpha \)-amino acids. Tetrahedron 65:9536–9541. doi:10.1016/j.tet.2009.09.045
Hamel E (2003) Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys 38:1–22. doi:10.1385/CBB:38:1:1
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Lesma, G., Sacchetti, A., Bai, R. et al. Hemiasterlin analogues incorporating an aromatic, and heterocyclic type C-terminus: design, synthesis and biological evaluation. Mol Divers 18, 357–373 (2014). https://doi.org/10.1007/s11030-014-9507-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9507-9