Abstract
Introduction
Opioid-sparing protocols reduce postpartum opioid prescribing in opioid-naïve patients; however, patients with opioid use disorder (OUD) and complex pain needs who may benefit from these protocols are typically excluded from them. We assessed postpartum pain experiences of patients with OUD and chronic prenatal opioid exposure after implementation of an opioid-sparing protocol.
Methods
A phone survey assessed postpartum pain experiences for people with chronic prenatal opioid exposure who delivered between January 2020 and August 2021 at an academic hospital. Analyses included descriptive statistics, qualitative content analysis, and a joint display comparing themes.
Results
Of 25 patients, 18 (72%) participated; most were non-Hispanic White (100%, 18/18), publicly insured (78%, 14/18), multiparous (78%, 14/18), with OUD (100%, 18/18). No patients with a vaginal birth received an opioid prescription; half (4/8) with a cesarean birth received one at discharge. Over one-third (7/18, 39%) reported poor pain control (≥ 5/10) in the hospital and one week post-discharge; scores were higher for cesarean versus vaginal birth. Qualitative sub-analyses of open-ended responses revealed patient perceptions of postpartum pain and treatment. The most effective strategies, stratified by birth type and pain level, ranged from non-opioid medications for vaginal births and minor pain to prescription opioids for cesarean births and moderate-to-intense pain.
Discussion
Postpartum opioid prescribing for patients with chronic prenatal opioid use was low for vaginal and cesarean birth following implementation of an opioid-sparing protocol. Patients with OUD reported good pain management with opioid-sparing pain regimens; however, many reported poorly controlled pain immediately postpartum. Future work should assess approaches to postpartum pain management that minimize the risks of opioid medication—particularly in at-risk groups.
Significance
What is already known on this subject? Opioid-sparing protocols can reduce postpartum opioid prescribing in opioid-naïve patients; however, there are currently no clear guidelines for opioid prescribing for people with opioid use disorder (OUD) in the postpartum period.
What this study adds?Postpartum opioid prescribing for patients with chronic prenatal opioid use was less than the national average and one-third of patients reported poor pain control. Opioid-sparing protocols postpartum should be expanded to patients with OUD to improve pain control and minimize risks associated with opioid medication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The opioid epidemic was originally linked to prescription opioids, which accounted for 70% of opioid overdose deaths between 1999 and 2008 (Centers for Disease Control & Prevention, 2011). In the past decade, opioid use disorder (OUD) in pregnancy increased by 131% and has been associated with increased maternal and neonatal morbidity and mortality (Hirai et al., 2021; Maeda et al., 2014). In light of the epidemic, there has been a significant focus on judicious prescribing of opioids—particularly after medical and surgical procedures (U.S. Department of Health & Human Services, 2019). Most of these efforts in the obstetric literature have focused on opioid-naïve patients with the goal of reducing new cases of OUD. There are currently no clear guidelines for opioid prescribing for people with OUD in the postpartum period.
Enhanced recovery after surgery programs have been implemented after a variety of surgical procedures to improve patients’ postoperative experience and outcomes through opioid-sparing protocols—increasing non-pharmacologic and non-opioid adjuncts for pain management that reduce postpartum opioid prescribing and reduce length of hospital stay in opioid-naïve patients (Shinnick et al., 2020). However, as patients with a history of OUD are typically excluded from these interventions, little is known about their use of such protocols and postpartum pain experiences.
Management of acute pain in this population is complex. Pain experiences may be underestimated due to stigma or bias and patients undertreated as a result (Alford et al., 2006). Special considerations for patients with OUD must include the potential development of tolerance and opioid-induced hyperalgesia—both resulting from prolonged opioid exposure (Angst & Clark, 2006; Soens et al., 2019). Patients maintained on medication for opioid use disorder (MOUD) have shown higher tolerance, requiring more opioids for acute postpartum pain than controls (American College of Obstetricians & Gynecologists, 2017). With opioid-induced hyperalgesia, patients may paradoxically experience more sensitivity to painful stimuli while being treated for pain. Given the unique aspects of pain management for this population, further exploration into the utility of opioid-sparing protocols to augment acute pain management are needed.
The primary objective of this study was to assess postpartum pain experiences of patients with OUD and chronic prenatal opioid exposure following implementation of an opioid-sparing protocol to better inform peripartum pain management for this population. Secondary objectives included understanding facilitators and barriers of effective postpartum pain management specific to this population and exploring perceived risk of opioid overdose. We hypothesized that use of non-pharmacologic interventions in patients with chronic prenatal opioid exposure would result in lower acute opioid use and improved pain experiences. All patients at our study site, including those with OUD, utilized opioid-sparing protocols postpartum, creating a unique opportunity to evaluate such protocols in this population.
Methods
We conducted phone surveys to assess postpartum pain management experiences of patients with a history of OUD who delivered at our Midwest academic medical center between January 2020 and August 2021. Our center initially implemented an opioid-sparing protocol in October 2017 that included opioid-sparing pain regimens, non-pharmacologic pain management strategies (e.g., deep breathing, hot packs), scheduled non-opioid medications, and judicious opioid prescribing. The protocol was enhanced with improved patient education and shared decision-making materials in July 2019. All components of the protocol were made available for patients with a history of OUD, though opioid prescribing limits were individually determined with the patient and their provider.
Patients were identified by ICD-9 and ICD-10 codes for OUD in pregnancy and MOUD use in pregnancy. Eligibility criteria for inclusion included delivering between January 2020 and August 2021, age ≥ 18, and reported chronic use of opioids for OUD throughout pregnancy. Patients received a $25 gift card for participation. This study was approved by our center’s institutional review board.
To capture our primary and secondary outcomes, we adapted a survey previously used to assess patient postoperative pain experiences (Anderson et al., 2021; Hallway et al., 2019; Howard et al., 2019). An interprofessional group consisting of a Maternal Fetal Medicine Specialist with expertise in OUD, an academic specialist in OBGYN with expertise in opioid-sparing protocols, and an expert in opioid-sparing protocols revised the survey instrument through a rapid iterative and collaborative process.
Survey domains including general demographics (e.g., age, parity); delivery characteristics (e.g., vaginal or cesarean birth); and postpartum information (e.g., breastfeeding initiation, decision to parent, separation from infant postpartum) were adapted from well-validated questionnaires designed for peripartum patients (Peahl et al., 2020; Roxanne et al., 2022). Patients were also queried about MOUD use.
To better understand postpartum pain management, patients were asked about their opioid consumption, non-opioid consumption, and postpartum pain management. Patients reported the total number of opioid tabs they consumed, their use of non-opioids (e.g., acetaminophen and ibuprofen), and if they used the “staggering” technique (i.e., alternating acetaminophen and ibuprofen). They also retrospectively reported pain scores (0 = no pain; 10 = worst pain) at two time points: during the childbirth hospitalization and from discharge to one week postpartum, and whether their pain was controlled without opioid medication. Patients reported how pain affected their ability to breastfeed, bond, and care for their infant using Likert scale responses (1 = strongly disagree; 5 = strongly agree) and answered open-ended questions about postpartum pain management, including treatments they deemed helpful postpartum or those that would have helped. Finally, participants were asked to rate the importance of a naloxone kit on a 5-point Likert scale (1 = not important; 5 = very important).
We refined the survey instrument with our institution’s interdisciplinary women’s healthcare effectiveness research program, then pilot-tested it with two patients and one social worker in our multidisciplinary substance use clinic for pregnant patients. Their responses were not included in the final analysis. Minor revisions to wording were incorporated following this initial pilot testing. The full survey is available in the Appendix.
The survey was uploaded to a REDCap database and study personnel contacted eligible participants, obtained verbal and electronic consent, read questions, and recorded answers in the database. To avoid reporting bias, all participants were interviewed by research assistants who were not involved in their prenatal or postpartum care.
In addition to data from the survey instrument, the electronic medical record was queried to confirm each patient’s history of OUD, maintenance on MOUD, mode of delivery, presence of an opioid prescription at hospital discharge, type of opioid and number of pills prescribed, indication of an opioid refill, anesthesia type used, pain score at hospital discharge (average of last three scores), and maternal and neonatal length of stay.
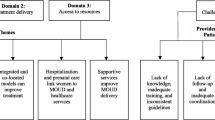
Given differences in pain associated with birth type, patients were grouped by vaginal versus cesarean birth. Maternal characteristics and delivery outcomes were derived from patient interviews as above. Quantitative data were summarized using basic descriptive statistics. All results, including number of respondents and proportions, were reported for the total number of patients who answered each question. A p-value < 0.05 was deemed statistically significant. Pearson’s correlation coefficient was calculated to measure the association between documented and patient-reported pain scores at hospital discharge. For qualitative analysis, patients were stratified by both birth type and reported pain control (< 5 vs. ≥ 5) in the seven days after hospital discharge to gain deeper understanding into the drivers of varying experiences. In total, there were four patient groupings: (1) vaginal birth with lower pain score; (2) vaginal birth with higher pain score; (3) cesarean birth with lower pain score; and (4) cesarean birth with higher pain score. Within these groups, open-ended survey responses were analyzed through content analysis, a method used to systematically classify textual data and identify common themes. Final analysis included the development of a joint display to compare emergent themes and representative quotes across stratified patient groups.
Results
Of 25 patients successfully contacted, 18 (72%) consented to participate. Patients were predominantly non-Hispanic White (18/18, 100%), publicly insured (14/18, 78%), and multiparous (14/18, 78%). Over half had a vaginal birth, one of which was complicated by postpartum hemorrhage managed with uterotonics (Table 1). There were no operative deliveries and no higher order perineal lacerations in this group. Only one of the eight cesarean births was unscheduled (secondary to non-reassuring fetal heart tones). All included patients had documented OUD, with 11/18 (61%) prescribed buprenorphine, 6/18 (33%) prescribed methadone, and 1/18 (6%) endorsing illicitly obtained opioid pills throughout pregnancy. Though most reported breastfeeding (12/18, 67%), this was the only statistically different postpartum outcome between groups, with patients undergoing vaginal birth reporting a significantly higher rate of breastfeeding than those with a cesarean birth (Table 1). There were no postpartum readmissions in this cohort.
No patients with a vaginal birth received a postpartum opioid prescription. Of those with a cesarean birth, 50% (4/8) received an opioid prescription at hospital discharge. The average prescription size was 78.8 (range 37.5–112.5) oral morphine equivalents (OME), equivalent to 10 tabs of 5 mg oxycodone. Half (2/4) of patients who received an opioid prescription required a refill (Table 2). Most patients utilized non-opioid pain medications for pain management, with 15/18 (83%) reporting acetaminophen and 13/18 (72%) reporting ibuprofen use after delivery; 11/18 (61%) reported using the staggering technique when taking both acetaminophen and ibuprofen. There were no differences in non-opioid medication use by mode of delivery (Table 2). One-third (7/18, 39%) of patients reported poor pain control (≥ 5/10) in the hospital and 1 week after discharge. Pain interfered with common parenting activities for patients, including breastfeeding (5/12, 42%), bonding with (6/18, 33.3%), and caring for (7/18, 39%) their newborn.
Our qualitative sub-analysis explored patients’ reported pain in the week postpartum and the strategies they employed to manage it (Table 3). Among patients undergoing vaginal birth, most reported lower pain scores (7/10, 70%). When they did experience mild pain in the week following discharge, non-opioid pain medications (e.g., acetaminophen, ibuprofen) were reported as helpful. One participant shared, “I did not have a lot of pain, so I did not need help managing it” (28-year-old, buprenorphine). Among patients who had a vaginal birth and experienced greater pain, 3/10 (30%) described how they utilized several pain management strategies, including non-opioid pain medication, topical agents, and cold compresses. One noted that she wanted additional pain relief, but felt “less heard after the baby was born” (28-year-old, buprenorphine/naloxone). While the strategies patients employed did not fully address their pain, these treatments did help make pain more manageable.
Patients with a cesarean birth reported greater average pain scores compared to those undergoing vaginal birth. Among those with a cesarean birth and lower pain scores, 4/8 (50%) remarked that the treatments administered in the hospital (e.g., epidurals, transversus abdominis plane block) were helpful in addressing their pain. However, these treatments were unavailable after hospital discharge as patients transitioned to other standard pain treatments (e.g., over-the-counter medication, heating pads, ice packs). One patient described the anesthesia as helping “a little bit with the pain but the side effect of the leg numbness was difficult to deal with. [I] would have preferred something else. Tylenol/Motrin were helpful” (28-year-old, buprenorphine). When asked about pain management, another patient reported that medications were helpful but went on to describe her more significant concern with how her medications (i.e., morphine and suboxone) were reported to Child Protective Services (CPS), after being told this information would not be shared. She remarked that, “it would have been more helpful if [I] would have known that the medications [I] was given could interfere with [my] life after the hospital” (28-year-old, buprenorphine/naloxone).
In the final sub-group of patients who had a cesarean birth and reported higher pain scores in the week following birth (4/8, 50%), three received oxycodone postpartum, which they reported as the most effective treatment for their pain. One patient remarked, “oral pain medications were helpful. If [I] hit the pain medication enough, it would relieve the pain at the incision” (33-year-old, methadone). However, she noted that, “once [I] stopped using oxycodone and other pain medications at home, it was difficult. [I] was thinking in the few days after finishing oxycodone, [I] could have gone up in the methadone to ease the pain [I] was feeling.”
We queried the last 10 patients interviewed about opioid overdose risk and prevention strategies. Regarding their personal risk of opioid overdose in the postpartum period, all (10/10, 100%) reported themselves as having “no risk at all.” Additionally, only 5/10 (50%) reported having access to a naloxone kit at home, while 8/10 (80%) reported knowing how to use naloxone. Regarding the importance of access to a naloxone kit, 5/10 (50%) thought it was “very important” and 5/10 (50%) deemed it “not important.”
Discussion
In a cohort of postpartum patients with a history of OUD, opioid prescribing rates were below the national average for opioid-naïve patients. Specifically, no patients received an opioid prescription after vaginal birth and 50% of patients received an opioid prescription after cesarean birth. Despite low rates of opioid prescribing in this population, one-third of patients described poor pain control in the hospital and one week following discharge. Many patients also reported that pain interfered with common postpartum parenting activities, including breastfeeding, caring for, and bonding with their newborn. Most of our cohort used and responded positively to opioid-sparing protocols for pain management, demonstrating their feasibility and acceptability in this population. Lastly, this high-risk group of patients perceived their risk of opioid overdose as low.
Qualitative analyses showed that non-opioid pain management strategies (e.g., acetaminophen, ibuprofen) were effective for patients who had a vaginal birth and lower pain levels, while those with greater pain levels suggested these strategies were helpful but insufficient. Among patients who had cesarean births, hospital-administered pain measures (e.g., epidural) were helpful for those with lower pain scores, as were opioid prescriptions for those with higher pain scores. However, patients who had cesarean births had concerns about long-ranging pain relief, as well as the sharing of their medication information and the impact on their lives.
Pregnant people with OUD frequently have negative experiences with pain management in pregnancy, including stigma, insufficient attention to their pain management needs, and poor access to recovery programs and resources (O'Rourke-Suchoff et al., 2020). Our study specifically expounded on the issues of opioid prescribing for postpartum patients with known OUD receiving pain management with an opioid-sparing protocol. Our findings further emphasize the importance of addressing and acknowledging pain of patients with chronic prenatal opioid exposure in the postpartum period and incorporating non-opioid adjuncts to support pain control.
Opioid-sparing pain regimens for cesarean birth have been associated with a reduction in inpatient and outpatient opioid prescribing and consumption with equivalent pain control (Hedderson et al., 2019). These protocols have also been linked to high patient satisfaction and decreased hospital length of stay (Lakhi et al., 2019; Shinnick et al., 2020). With approximately one in three births in the U.S. occurring via cesarean, there is an increased need to include patients with OUD in opioid-sparing protocols (Bateman et al., 2016; Peahl et al., 2019). Although patients with a history of OUD have been historically excluded from opioid-sparing interventions, our findings show that most patients with OUD found non-opioid pain management interventions and strategies helpful in managing their acute pain. In fact, our cohort had lower rates of opioid prescribing compared to contemporary opioid-naïve cohorts (0% vs. 27% for vaginal birth and 50% vs. 75% for cesarean birth) (Peahl et al., 2019). These findings suggest that patients with OUD fill postpartum opioid prescriptions at lower rates than an opioid-naïve population.
Lower prescribing for patients with OUD may have various explanations, including clinicians being reluctant to prescribe opioids for these patients and patients exhibiting “self-stigma” and having concerns about receiving or requesting an opioid prescription. Alternatively, patients may actively choose to pursue non-opioid pain control methods to avoid increased opioid exposure due to continued awareness and agency in their own health.
While lower opioid prescribing may be good or appropriate, there are potential harms to untreated pain during birth. Insufficient pain management may result in patients “not feeling heard,” as expressed by one of our participants. Additionally, for postpartum patients with OUD, adequate pain management may be linked to broader concerns related to drug- and treatment-related stigma and discrimination, including CPS involvement and fears of losing custody of their children (Falletta et al., 2018). These negative experiences may hinder patients from seeking pain medications, potentially impacting their recovery, interfering with their ability to parent, or increasing their chance of relapse. Our findings support other researchers’ calls for consistent, transparent, and non-judgmental communication from providers during the entire peripartum and postpartum course to break down barriers to care-seeking (Goodman et al., 2020).
We found low perceived risk of opioid overdose in our high-risk population. Previous research in a population of adult illicit opioid users showed that those who inject more frequently and older users were less likely to perceive themselves as being at risk for overdose (Rowe et al., 2016). Although perceptions regarding the importance of naloxone access were mixed, the majority of participants understand how to use this life-saving medication. Pregnancy is often a time when patients with OUD are motivated to start substance use treatment or MOUD. However, opioid overdose contributes to 5.6% of all pregnancy-associated deaths, with some studies showing rates upwards of 11–20% (Mitra et al.,2020; Schiff et al., 2018). More work is needed to understand the discrepancy in patients’ and healthcare systems’ perceptions of opioid overdose risks.
Our findings emphasize the importance of including patients with chronic prenatal opioid exposure or on MOUD in planned opioid-sparing interventions. While prescription opioids proved effective for patients with significant pain after cesarean birth, more education to prepare patients for birth, strategies to cope with pain, and options for acute pain management should be readily available. O’Rourke et al. described the importance of providing pregnant people with OUD early anticipatory guidance on pain management in the setting of MOUD, as well as arranging prenatal consults with anesthesiologists. This shared decision-making framework could decrease anxiety and improve healthcare professional trust among birthing people with OUD (Alford et al., 2006).
Patients’ experiences also highlight an opportunity for better coordination of treatment plans prenatally for pain management and OUD during the postpartum period, including coordinating prescribing transitions for MOUD, understanding the role of CPS in care, mandated reporting, and the benefit of opioid-sparing protocols. This will allow providers to address broader concerns related to drug- and treatment-related stigma and discrimination through early, open communication and continued nonjudgmental care.
Our study highlights several key areas for future exploration. First, there is a critical need to understand discrepancies in patients’ reported pain control, pain scores, and medication use. We noted lower opioid prescribing in our cohort compared to the national average, with most patients responding positively to opioid-sparing protocols for pain management. Despite positive comments about pain management with non-opioid adjuncts, we noted a discrepancy between pain scores and patient commentary (good pain control, but high pain scores reported). Patients did not use pain medication even when they had pain. It is possible that our experienced, mostly multiparous population had a better understanding of what level of pain to expect with delivery, and although they rated their pain as intermediate, it may have been more manageable than previous deliveries. Additionally, only half of our cohort with cesarean births received postpartum opioid prescriptions. Further research is needed to understand reasons patients with OUD accept or decline opioid prescriptions after cesarean birth.
We also found that pain affected several aspects of postpartum recovery and parenting, including breastfeeding, infant care, and bonding. Understanding how pain limits these important activities in the immediate postpartum period is essential for best supporting pregnant people and their infants. Lastly, MOUD type may impact pain perceptions and should be further explored in a larger study.
Strengths and Limitations
Our study is one of the first to investigate postpartum pain perceptions in a population of birthing people with OUD. Despite the time between delivery and survey (from 6 weeks to 12 months), documented and patient-reported pain scores at hospital discharge were strongly correlated, supporting the validity of our results. Additionally, this study expands on perceptions of birthing people with chronic prenatal opioid exposure and their experiences with healthcare teams, breastfeeding, and newborn bonding and pain management after delivery (Bateman et al., 2016; Falletta et al., 2018; Peahl et al., 2019). While prior studies have excluded patients with OUD from opioid-sparing protocols, our study and institution successfully included birthing people with OUD in this effective, patient-centered intervention. Our study provides early data on pain outcomes in this understudied population.
Our study does have limitations. First, we were limited to phone interviews due to COVID-19 restrictions, which limited the number of participants we were able to successfully contact. Additionally, our participant sample was racially homogenous, with all participants identifying as non-Hispanic White. As the majority of included patients were multiparous, it is possible that effective methods of pain control were already established in previous pregnancies. Social determinants of health and history of trauma were not collected and may have impacted reported pain scores. In light of emerging national data highlighting maternal overdose as a leading cause of maternal mortality, we sought to assess overdose risk in our study population. Changes in our survey during the COVID-19 pandemic and increased time for IRB review delayed implementation of these questions. This resulted in few participants being queried about opioid overdose risk and prevention, making this data less generalizable.
Conclusions
Our study’s findings call on OBGYNs, anesthesiologists, nurses, and other maternal health providers to prioritize patient-centered care and education for the unique population of birthing people with OUD. Our data suggests that non-opioid pain medication and adjuncts were highly utilized by this population and were felt to be effective, with few patients filling opioid prescriptions postpartum. However, patients did report that pain interfered with breastfeeding, bonding, and other infant care—emphasizing the importance of adequate pain control in this population postpartum. Increased awareness and inclusion of people with OUD in pain management discussions is crucial to mitigating stigma, discrimination, and lack of education surrounding these topics. Larger studies of patients from more diverse backgrounds are needed to further explore pain perceptions.
Data Availability
Available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Alford, D. P., Compton, P., & Samet, J. H. (2006). Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Annals of Internal Medicine, 144(2), 127–134. https://doi.org/10.7326/0003-4819-144-2-200601170-00010
American College of Obstetricians and Gynecologists. (2017). Committee opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstetrics & Gynecology, 130(2), e81–e94. https://doi.org/10.1097/AOG.0000000000002235
Anderson, M., Hallway, A., Brummett, C., Waljee, J., Englesbe, M., & Howard, R. (2021). Patient-reported outcomes after opioid-sparing surgery compared with standard of care. JAMA Surgery, 156(3), 286–287. https://doi.org/10.1001/jamasurg.2020.5646
Angst, M. S., & Clark, J. D. (2006). Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology, 104(3), 570–587. https://doi.org/10.1097/00000542-200603000-00025
Bateman, B. T., Franklin, J. M., Bykov, K., Avorn, J., Shrank, W. H., Brennan, T. A., Landon, J. E., Rathmell, J. P., Huybrechts, K. F., Fischer, M. A., & Choudhry, N. K. (2016). Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naive women. American Journal of Obstetrics and Gynecology, 215(3), 353 e1-353 e18. https://doi.org/10.1016/j.ajog.2016.03.016
Centers for Disease Control and Prevention. (2011). Vital signs: Overdoses of prescription opioid pain relievers–-United States, 1999–2008. Morbidity and Mortality Weekly Report, 60(43), 1487–1492.
Falletta, L., Hamilton, K., Fischbein, R., Aultman, J., Kinney, B., & Kenne, D. (2018). Perceptions of child protective services among pregnant or recently pregnant, opioid-using women in substance abuse treatment. Child Abuse and Neglect, 79, 125–135. https://doi.org/10.1016/j.chiabu.2018.01.026
Goodman, D. J., Saunders, E. C., & Wolff, K. B. (2020). In their own words: A qualitative study of factors promoting resilience and recovery among postpartum women with opioid use disorders. BMC Pregnancy and Childbirth, 20(1), 178. https://doi.org/10.1186/s12884-020-02872-5
Hallway, A., Vu, J., Lee, J., Palazzolo, W., Waljee, J., Brummett, C., Englesbe, M., & Howard, R. (2019). Patient satisfaction and pain control using an opioid-sparing postoperative pathway. Journal of the American College of Surgeons, 229(3), 316–322. https://doi.org/10.1016/j.jamcollsurg.2019.04.020
Hedderson, M., Lee, D., Hunt, E., Lee, K., Xu, F., Mustille, A., Galin, J., Campbell, C., Quesenberry, C., Reyes, V., Huang, M., Nicol, B., Paulson, S., & Liu, V. (2019). Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: A quality improvement initiative. Obstetrics & Gynecology, 134(3), 511–519. https://doi.org/10.1097/AOG.0000000000003406
Hirai, A. H., Ko, J. Y., & Patrick, S. W. (2021). US hospital data about neonatal abstinence syndrome and maternal opioid-related diagnoses-reply. JAMA, 325(20), 2120. https://doi.org/10.1001/jama.2021.4519
Howard, R., Fry, B., Gunaseelan, V., Lee, J., Waljee, J., Brummett, C., Campbell, D., Jr., Seese, E., Englesbe, M., & Vu, J. (2019). Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surgery, 154(1), e184234. https://doi.org/10.1001/jamasurg.2018.4234
Lakhi, N., Tricorico, G., Kanninen, T., Suddle, R., Ponterio, J., & Moretti, M. (2019). Post-cesarean delivery outpatient opioid consumption and perception of pain control following implementation of a restrictive opioid prescription protocol. American Journal of Obstetrics & Gynecology MFM, 1(4), 100049. https://doi.org/10.1016/j.ajogmf.2019.100049
Maeda, A., Bateman, B. T., Clancy, C. R., Creanga, A. A., & Leffert, L. R. (2014). Opioid abuse and dependence during pregnancy: Temporal trends and obstetrical outcomes. Anesthesiology, 121(6), 1158–1165. https://doi.org/10.1097/ALN.0000000000000472
Mitra, A., Brandt, J., Rosen, T., Ananth, C., & Schuster, M. (2020). Opioid use disorder: A poorly understood cause of maternal mortality in the United States [26E]. Obstetrics & Gynecology, 135(Suppl 1), 56S. https://doi.org/10.1097/01.AOG.0000663364.06637.15
O’Rourke-Suchoff, D., Sobel, L., Holland, E., Perkins, R., Saia, K., & Bell, S. (2020). The labor and birth experience of women with opioid use disorder: A qualitative study. Women Birth, 33(6), 592–597. https://doi.org/10.1016/j.wombi.2020.01.006
Peahl, A. F., Dalton, V. K., Montgomery, J. R., Lai, Y. L., Hu, H. M., & Waljee, J. F. (2019). Rates of new persistent opioid use after vaginal or cesarean birth among US women. JAMA Network Open, 2(7), e197863. https://doi.org/10.1001/jamanetworkopen.2019.7863
Peahl, A. F., Novara, A., Heisler, M., Dalton, V. K., Moniz, M. H., & Smith, R. D. (2020). Patient preferences for prenatal and postpartum care delivery: A survey of postpartum women. Obstetrics & Gynecology, 135(5), 1038–1046. https://doi.org/10.1097/AOG.0000000000003731
Rowe, C., Santos, G. M., Behar, E., & Coffin, P. O. (2016). Correlates of overdose risk perception among illicit opioid users. Drug and Alcohol Dependence, 159, 234–239. https://doi.org/10.1016/j.drugalcdep.2015.12.018
Roxanne, B., Laura, V. D. B., Yannic, V. G., Natacha, V. C., Luka, V. L., & Kuipers, Y. J. (2022). Validation of the postpartum bonding questionnaire: A cross-sectional study among Flemish mothers. Midwifery, 107, 103280. https://doi.org/10.1016/j.midw.2022.103280
Schiff, D. M., Nielsen, T., Terplan, M., Hood, M., Bernson, D., Diop, H., Bharel, M., Wilens, T. E., LaRochelle, M., Walley, A. Y., & Land, T. (2018). Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstetrics & Gynecology, 132(2), 466–474. https://doi.org/10.1097/AOG.0000000000002734
Shinnick, J. K., Ruhotina, M., Has, P., Kelly, B. J., Brousseau, E. C., O’Brien, J., & Peahl, A. F. (2020). Enhanced recovery after surgery for cesarean delivery decreases length of hospital stay and opioid consumption: A quality improvement initiative. American Journal of Perinatology. https://doi.org/10.1055/s-0040-1709456
Soens, M. A., He, J., & Bateman, B. T. (2019). Anesthesia considerations and post-operative pain management in pregnant women with chronic opioid use. Seminars in Perinatology, 43(3), 149–161. https://doi.org/10.1053/j.semperi.2019.01.004
U.S. Department of Health and Human Services. (2019). HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics. Retrieved November 15, 2022 from https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
Acknowledgements
We would like to thank all of our participants for sharing their postpartum experiences. We would also like to thank Sarah Block for her assistance with manuscript preparation.
Funding
Funding for this research was provided by the National Center for Advancing Translational Sciences through the Michigan Institute for Clinical & Health Research (MICHR UL1TR002240).
Author information
Authors and Affiliations
Contributions
The authors were involved as follows: CT, CS, AP, JW, and KJ made substantial contributions to conception and design; SI, BN, AH, and CT participated in data acquisition; KJ, CT, CJ, and AH performed the analysis and interpretation of data; CT, SI, KJ, CS, JW, AH, and AP were involved in drafting the manuscript and revising it critically for important intellectual content. All authors have given final approval of the version to be published. Each author has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any.
Corresponding author
Ethics declarations
Competing Interests
The authors report no conflicts of interest.
Ethical Approval
This study was approved by the University of Michigan Institutional Review Board (HUM00172278).
Consent to Participate
Study personnel obtained verbal and electronic consent from participants, read questions, and recorded answers in a secure REDCap database. To avoid reporting bias, all participants were interviewed by research assistants who were not involved in their prenatal or postpartum care.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Townsel, C., Irani, S., Nguyen, BH. et al. Use of Opioid-Sparing Protocols and Perceived Postpartum Pain in Patients with Opioid Use Disorder and Chronic Prenatal Opioid Exposure. Matern Child Health J 27, 1416–1425 (2023). https://doi.org/10.1007/s10995-023-03710-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-023-03710-8