Abstract
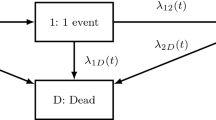
Recurrent events refer to events that over time can occur several times for each individual. Full use of such data in a clinical trial requires a method that addresses the dependence between events. For modelling this dependence, there are two time scales to consider, namely time since start of the study (running time) or time since most recent event (gap time). In the multi-state setup, it is possible to estimate parameters also in the case, where the hazard model allows for an effect of both time scales, making this an extremely flexible approach. However, for summarizing the effect of a treatment in a transparent and informative way, the choice of time scale and model requires much more care. This paper discusses these choices both from a theoretical and practical point of view. This is supported by a simulation study showing that in a frailty model with assumptions covered by both time scales, the gap time approach may give misleading results. A literature dataset is used for illustrating the issues.









Similar content being viewed by others
References
Aalen O, Cook R, Røysland K (2015) Does cox analysis of a randomized survival study yield a causal treatment effect? Lifetime Data Anal 21:579–593
Aalen O, Johansen S (1978) An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scand J Statist 5:141–150
Cook R, Lawless J (2007) The statistical analysis of recurrent events. Springer-Verlag, Berlin
Fritsch A, Schlomer P, Mendolia F, Mutze T, Jahn-Eimermacher A, Roger J (2021) Efficiency comparison of analysis methods for recurrent event and time-to-first-event endpoints application to clinical trials in chronic heart failure. Statist Biopharm Res (online early). https://doi.org/10.1080/19466315.2021.1945488
Gonzalez J, Fernandez E, Moreno V, Ribes J, Peris M, Navarro M, Cambray M, Borras J (2005) Sex differences in hospital readmission among colorectal cancer patients. J Epidemiol Community Health 59:506–11
Hougaard P (2000) Analysis of multivariate survival data. Springer-Verlag, Berlin
ICH (2019) Addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials E9(R1), International Council for Harmonisation
Lawless J, Nadeau C (1995) Some simple robust methods for the analysis of recurrent events. Technometrics 37:158–168
Lin D, Wei L (1989) The robust inference for the cox proportional hazards model. J Am Statist Assoc 84:1074–1078
Lin D, Wei L, Yang I, Ying Z (2000) Semiparametric regression for the mean and rate functions of recurrent events. J R Statist Soc B 62:711–730
Oakes D (1982) A model for association in bivariate survival data. J R Statist Soc B 44:414–422
Oakes D (1989) Bivariate survival models induced by frailties. J Am Statist Assoc 84:487–493
Prentice R, Williams B, Peterson A (1981) On the regression analysis of multivariate failure time data. Biometrika 68:373–379
Rondeau V, Mazroui Y, Gonzalez J (2012) frailtypack: an r package for the analysis of correlated survival data with frailty models using penalized likelihood estimation or parametrical estimation. J Statist Softw 47:1–28
Schmidli H, Roger J, Akacha M (2021) Estimands for recurrent event endpoints in the presence of a terminal event. Statist Biopharm Res (online early). https://doi.org/10.1080/19466315.2021.1895883
Therneau T, Grambsch P (2000) Modeling survival data. Springer-Verlag, Berlin
Wei J, Mutze T, Jahn-Eimermacher A, Roger J (2021) Properties of two while-alive estimands for recurrent events and their potential estimators. Statist Biopharm Res (online early). https://doi.org/10.1080/19466315.2021.1994457
Acknowledgements
The comments from Henrik Ravn on an earlier version of this paper are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hougaard, P. Choice of time scale for analysis of recurrent events data. Lifetime Data Anal 28, 700–722 (2022). https://doi.org/10.1007/s10985-022-09569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10985-022-09569-1