Abstract
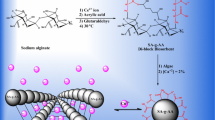
Novel amino-carbamate moiety grafted calcium alginate hydrogel beads (CA-1) were synthesized by reacting sodium alginate with 4-phenylsemicarbazide followed by ionotropic crosslinking with Ca(II) ions. As compared to pure calcium alginate hydrogel beads (CA), CA-1 exhibited fast kinetics and enhanced sorption capacity towards U(VI) ions, from mild acidic conditions. The sorption kinetic could be described by pseudo-second order equation, with the chemisorption as the rate-controlling step. The sorption isotherm were fitted well by Langmuir (qm = 233.2 mg/g at 298 K). CA-1 hydrogel beads exhibited fast kinetic, high sorption capacity and excellent selectivity for U(VI) sorption, thus it could be potentially used for the removal/recovery of U(VI) ions from wastewater.










Similar content being viewed by others
References
Havelka S, Berak L, Kourim V, Peka I, Podest M (1973) Research on fuel cycles of nuclear power stations carried out in the chemistry department of the institute of nuclear research. Stat Neerl 11:63–76
Aly MM, Hamza MF (2013) A review: studies on uranium removal using different techniques. Overview. J Disper Sci Technol 34:182–213
Elwakeel KZ, Atia AA (2014) Uptake of U(VI) from aqueous media by magnetic Schiff’s base chitosan composite. J Clean Prod 70:292–302
Galhoum AA, Mahfouz MG, Atia AA (2015) Amino acid functionalized chitosan magnetic nanobased particles for uranyl sorption. Ind Eng Chem Res 54:12374–12385
Kim JS, Lee JY, Yoon HS, Kumar JR (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Method 40:77–125
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Read AGH, Miura N, Carter JL et al (2018) Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Int J Biol Macromol 117:78–85
Chen JP, Kuo CY, Lee WL (2012) Thermo-responsive wound dressings by grafting chitosan and poly(n-isopropylacrylamide) to plasma-induced graft polymerization modified non-woven fabrics. Appl Surf Sci 262:95–101
Iftekhar S, Srivastava V, Hammouda SB, Sillanpää M (2018) Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr Polym 194:274–284
Cui J, Zhou Z, Liu S et al (2018) Synthesis of cauliflower-like ion imprinted polymers for selective adsorption and separation of lithium ion. New J Chem 42:14502–14509
Sone H, Fugetsu B, Tanaka S (2009) Selective elimination of lead(II) ions by alginate/polyurethane composite foams. J Hazard Mater 162:423–429
Wu J, Tian K, Wang J et al (2018) Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem Eng J 106:124–137
Shehzad H, Zhou L, Li Z, Chen Q, Wang Y, Liu Z (2018) Effective adsorption of U(VI) from aqueous solution using magnetic chitosan nanoparticles grafted with maleic anhydride: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 315:195–206
Yu S, Dai Y, Cao X (2016) Adsorption of uranium(VI) from aqueous solution using a novel magnetic hydrothermal cross-linking chitosan. J Radioanal Nucl Chem 310:651–660
Sheng L, Zhou L, Huang Z (2016) Facile synthesis of magnetic chitosan nano-particles functionalized with N/O-containing groups for efficient adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 310:1361–1371
Wang G, Liu J, Wang X (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Yu J, Wang J, Jiang Y (2017) Removal of uranium from aqueous solution by alginate beads. Nucl Eng Technol 49:534–540
Fan J, Shi Z, Lian M, Li H, Yin J (2013) Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J Mater Chem 1:7433–7439
Ahmad A, Bhat AH, Buang A (2018) Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: kinetic and equilibrium modeling. J Clean Prod 171:1361–1375
Papageorgiou SK, Katsaros FK, Kouvelos EP, Nolan JW, Deit HL, Kanellopoulos NK (2006) Heavy metal sorption by calcium alginate beads from laminaria digitata. J Hazard Mater 137:1765–1772
Shengye W, Thierry V, Catherine F, Eric G (2016) Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int J Mol Sci 17:1453–1460
Vijayalakshmi K, Gomathi T (2014) Preparation and characterization of nanochitosan/sodium alginate/microcrystalline cellulose beads. Pharm Chem 6:65–77
Gotoh T, Matsushima K, Kikuchi KI (2004) Preparation of alginate-chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55:135–140
Benettayeb A, Guibal E, Morsli A, Kessas R (2017) Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem Eng J 316:704–714
Leal D, Matsuhiro B, Rossi M, Caruso F (2008) FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr Res 343:308–316
Şimşek S, Yilmaz E, Boztu A (2013) Amine-modified maleic anhydride containing terpolymers for the adsorption of uranyl ion in aqueous solutions. J Radioanal Nucl Chem 298:923–930
Ouyang J, Wang Y, Li T, Zhou L, Liu Z (2018) Immobilization of carboxyl-modified multiwalled carbon nanotubes in chitosan-based composite membranes for U(VI) sorption. J Radioanal Nucl Chem 317:1419–1428
Zhou L, Zou H, Wang Y, Liu Z, Adesina AA (2016) Immobilization of in situ generated Fe0-nanoparticles in tripolyphosphate-crosslinking chitosan membranes for enhancing U(VI) sorption. J Radioanal Nucl Chem 311:1–9
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Handlingar 24:1–39
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper(II) on to peat. Adsorpt Sci Technol 20:797–815
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold (III) and silver (I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87:197–206
Yang S, Qian J, Kuang L, Hua D (2017) Ion-imprinted mesoporous silica for selective removal of uranium from highly acidic and radioactive effluent. ACS Appl Mater Int 9:29337–29344
Basu H, Singhal RK, Pimple MV, Saha S (2018) Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J Environ Chem Eng 6:1625–1633
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO2 2+ from aqueous solution. J Phys Chem B 113:860–864
Kim JH, Lee HI, Yeon JW, Jung Y, Kim JM (2010) Removal of uranium(VI) from aqueous solutions by nanoporous carbon and its chelating polymer composite. J Radioanal Nucl Chem 286:129–133
Shao D, Wang X, Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41:6182–6188
Zhao Y, Li J, Zhang S, Chen H, Shao D (2013) Efficient enrichment of uranium(VI) on amidoximated magnetite/graphene oxide composites. RSC Adv 3:18952–18959
Acknowledgements
The work is financially supported by the National Natural Science Foundation (21667001; 21866002; 21866005; 21706028; 21866006), the Key Research and Development Program and the Natural Science Fund Program of Jiangxi Province (20161BBF60059; S2017ZRMSB0473).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shehzad, H., Zhou, L., Wang, Y. et al. Effective biosorption of U(VI) from aqueous solution using calcium alginate hydrogel beads grafted with amino-carbamate moieties. J Radioanal Nucl Chem 321, 605–615 (2019). https://doi.org/10.1007/s10967-019-06631-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06631-5