Abstract
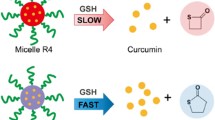
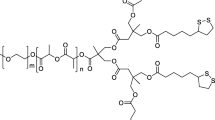
In this study, we prepared nanogels of a disulfide-cleavable polymer via polyionic complexation and genipin cross-linking and evaluated their reduction-triggered intracellular curcumin (Cur) delivery. These nanogels were stable at physiological conditions due to the formation of genipin cross-linking and helical PLL/PDC complexes and would swell/dissociate at acidic and reductive conditions due to the dissociation of PLL/PDC complexes and cleaving of disulfide bonds. The cellular uptake and intracellular release of Cur-loaded nanogels were demonstrated by tracking the fluorescent Cur and in vitro drug release studies, confirming the triggered release of Cur at acidic and reductive microenvironments in cells. The MTT, TUNEL staining, and Caspase-3 activity assays showed that the Cur-loaded nanogels exhibited higher cellular proliferation inhibition toward U-87 MG cells than free Cur, whereas the blank nanogels exhibited low cytoxicity. The results highlight the potential of functional nanogels prepared by polyionic complexation and cross-linking as a smart nanocarrier for drug delivery.








Similar content being viewed by others
References
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–131
Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z (2011) Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152:2–12
Torchilin V (2011) Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–135
Attia ABE, Ong ZY, Hedrick JL, Lee PP, Ee PLR, Hammond PT, Yang YY (2011) Mixed micelles self-assembled from block copolymers for drug delivery. Curr Opin Colloid Interface Sci 16:182–194
Carlsen A, Lecommandoux S (2009) Self-assembly of polypeptide-based block copolymer amphiphiles. Curr Opin Colloid Interface Sci 14:329–339
He C, Zhuang X, Tang Z, Tian H, Chen X (2012) Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv Healthc Mater 1:48–78
Voets IK, de Keizer A, Cohen Stuart MA (2009) Complex coacervate core micelles. Adv Colloid Interf Sci 147-148:300–318
Lee Y, Kataoka K (2009) Biosignal-sensitive polyion complex micelles for the delivery of biopharmaceuticals. Soft Matter 5:3810–3817
Zhao L, Li N, Wang K, Shi C, Zhang L, Luan Y (2014) A review of polypeptide-based polymersomes. Biomaterials 35:1284–1301
O'Reilly RK, Hawker CJ, Wooley KL (2006) Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem Soc Rev 35:1068–1083
Read ES, Armes SP (2007) Recent advances in shell cross-linked micelles. Chem Commun (29):3021–3035. doi:10.1039/B701217A
van Nostrum CF (2011) Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter 7:3246–3259
Du JZ, O'reilly RK (2009) Advances and challenges in smart and functional polymer vesicles. Soft Matter 5:3544–3561
Meng F, Zhong Z, Feijen J (2009) Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 10:197–209
Jackson AW, Fulton DA (2013) Making polymeric nanoparticles stimuli-responsive with dynamic covalent bonds. Polym Chem 4:31–45
Lee ES, Gao Z, Bae YH (2008) Recent progress in tumor pH targeting nanotechnology. J Control Release 132:164–170
Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB (2002) Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 62:307–312
Bae Y, Fukushima S, Harada A, Kataoka K (2003) Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed 42:4640–4643
Dai S, Ravi P, Tam KC (2008) pH-responsive polymers: synthesis, properties and applications. Soft Matter 4:435–449
Huang YC, Arham M, Jan JS (2013) Bioactive vesicles from saccharide- and hexanoyl-modified poly(L-lysine) copolypeptides and evaluation of the cross-linked vesicles as carriers of doxorubicin for controlled drug release. Euro Polym J 49:726–737
Chen BY, Huang YF, Huang YC, Wen TC, Jan JS (2014) Alkyl chain-grafted poly(L-lysine) vesicles with tunable molecular assembly and membrane permeability. ACS Macro Lett 3:220–223
Wang SSS, How SC, Chen YD, Tsai YH, Jan JS (2015) Bioactive saccharide-conjugated polypeptide micelles for acid-triggered doxorubicin delivery. J Mater Chem B 3:5220–5231
Du JZ, Du XJ, Mao CQ, Wang J (2011) Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc 133:17560–17563
Cheng J, Ji R, Gao SJ, Du FS, Li ZC (2012) Facile synthesis of acid-labile polymers with pendent ortho esters. Biomacromolecules 13:173–179
Guo X, Shi C, Wang J, Di S, Zhou S (2013) pH-triggered intracellular release from actively targeting polymer micelles. Biomaterials 34:4544–4554
Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z (2009) Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 30:6358–6366
Wen HY, Dong HQ, Xie WJ, Li YY, Wang K, Pauletti GM, Shi DL (2011) Rapidly disassembling nanomicelles with disulfide-linked PEG shells for glutathione-mediated intracellular drug delivery. Chem Commun 47:3550–3552
Liu J, Pang Y, Huang W, Zhu Z, Zhu X, Zhou Y, Yan D (2011) Redox-responsive polyphosphate nanosized assemblies: a smart drug delivery platform for cancer therapy. Biomacromolecules 12:2407–2415
Lee MH, Yang Z, Lim CW, Lee YH, Dongbang S, Kang C, Kim JS (2013) Disulfide-cleavage-triggered Chemosensors and their biological applications. Chem Rev 113:5071–5109
Ding J, Xu W, Zhang Y, Sun D, Xiao C, Liu D, Zhu X, Chen X (2013) Self-reinforced endocytoses of smart polypeptide nanogels for “on-demand” drug delivery. J Control Release 152:2–12
Huang K, Shi B, Xu W, Ding J, Yang Y, Liu H, Zhuang X, Chen X (2015) Reduction-responsive polypeptide nanogel delivers antitumor drug for improved efficacy and safety. Acta Biomater 27:179–193
Liu X, Wang J, Xu W, Ding J, Shi B, Huang K, Zhuang X, Chen X (2015a) Glutathione-degradable drug-loaded nanogel effectively and securely suppresses hepatoma in mouse model. Int J Nanomedicine 10:6587–6602
Yallapu MM, Jaggi M, Chauhan SC (2012) Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 17:71–80
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB (2008) Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett 267:133–164
Gou M, Men K, Shi H, Xiang M, Zhang J, Song J, Long J, Wan Y, Luo F, Zhao X, Qian Z (2011) Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 3:1558–1567
Zhao J, Liu J, Xu S, Zhou J, Han S, Deng L, Zhang J, Liu J, Meng A, Dong A (2013) Graft copolymer nanoparticles with pH and reduction dual-induced disassemblable property for enhanced intracellular curcumin release. ACS Appl Mater Interfaces 5:13216–13226
Cai M, Zhu K, Qiu Y, Liu X, Chen Y, Luo X (2014) pH and redox-responsive mixed micelles for enhanced intracellular drug release. Colloids Surf B Biointerfaces 116:424–431
Lee Y, Ishii T, Cabral H, Kim HJ, Seo JH, Nishiyama N, Oshima H, Osada K, Kataoka K (2009) Charge-conversional polyionic complex micelles-efficient nanocarriers for protein delivery into cytoplasm. Angew Chem Int Ed 48:5309–5312
Liu ST, Tuan-Mu HY, Hu JJ, Jan JS (2015b) Genipin cross-linked PEG-block-poly(l-lysine)/disulfide-based polymer complex micelles as fluorescent probes and pH−/redox-responsive drug vehicles. RSC Adv 5:87098–87107
Ding J, Shi F, Xiao C, Lin L, Chen L, He C, Zhuang X, Chen X (2011) One-step preparation of reduction-responsive poly(ethylene glycol)-poly(amino acid)s nanogels as efficient intracellular drug delivery platforms. Polym Chem 2:2857–2864
Shi F, Ding J, Xiao C, Zhuang X, He C, Chen L, Chen X (2012) Intracellular microenvironment responsive PEGylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J Mater Chem 22:14168–14179
Mohs AM, Wang X, Goodrich KC, Zong Y, Parker DL, Lu ZR (2004) PEG-g-poly(GdDTPA-co-L-cystine): a biodegradable macromolecular blood pool contrast agent for MR imaging. Bioconjug Chem 15:1424–1430
Huang YC, Arham M, Jan JS (2011) Alkyl chain grafted poly(l-lysine): self-assembly and biomedical application as carriers. Soft Matter 7:3975–3983
Hsieh YH, Hsiao YT, Jan JS (2014) Shell and core cross-linked poly(L-lysine)/poly(acrylic acid) complex micelles. Soft Matter 10:9568–9576
Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, Lei XH, Sun X, Liu X, Wang HB, Li XF, Yang DB, Sun Y, Zhao ZF, Jiang T, Li YL, Jiang CL (2013) Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Ther 19:926–936
Green JC, Reed DR (1998) Mitochondria and apoptosis. Science 281:1309–1312
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Sikora E, Bielak-Zmijewska A, Magalska A, Piwocka K, Mosieniak G, Kalinowska M, Widlak P, Cymerman IA, Bujnicki JM (2006) Curcumin induces caspase-3-dependent apoptotic pathway but inhibits DNA fragmentation factor 40/caspase-activated DNase endonuclease in human Jurkat cells. Mol Cancer Ther 5:927–934
Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB (2002) Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 23:143–150
Acknowledgements
The authors thank Miao-Er Chien for her support on the experiments and the funding supports from Ministry of Science and Technology Taiwan (MOST 104-2811-E-006-243 and 105-2221-E-006-248) and Show Chwan Memorial Hospital.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 1496 kb)
Rights and permissions
About this article
Cite this article
Lee, PY., Tuan-Mu, HY., Hsiao, LW. et al. Nanogels comprising reduction-cleavable polymers for glutathione-induced intracellular curcumin delivery. J Polym Res 24, 66 (2017). https://doi.org/10.1007/s10965-017-1207-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1207-6