Abstract
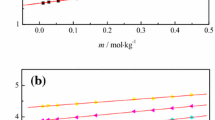
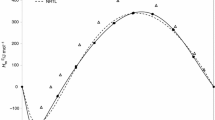
Enthalpies of solution and apparent molar volumes have been determined for propionamide in aqueous methanol, ethanol and propanol solutions at 298.15 K using a C-80 microcalorimeter and a DMA60/602 vibrating-tube digital densimeter. The enthalpic and volumetric interaction coefficients have been calculated. Using the present results along with results from previous studies for formamide, the pair-interaction coefficients are discussed from the perspective of dipole-dipole and structural interactions. In addition, the triplet interaction coefficients are interpreted by using the solvent-separated association mechanism.
Similar content being viewed by others
References
Lilley, T.H.: Interactions in solutions: The interplay between solute solvation and solute-solute interactions. Pure Appl. Chem. 66, 429–434 (1994)
Lilley, T.H.: The interplay between solute solvation and solute-solute interactions in solutions containing amino acids, peptides and related species. Pure Appl. Chem. 65, 2551–2560 (1993)
Von Hippel, P.H., Wang, K.Y.: On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J. Biol. Chem. 240, 3909–3923 (1965)
Chien-Chyon, W.C., Vois, A., Frank, J.: Protonation of amides in a helix-breaking solvent. J. Am. Chem. Soc. 86, 4774–4778 (1964)
Lu, Y., Wang, X.F., Su, G.J.: Calorimetric and volumetric studies of the interactions of formamide with alkan-1-ol in water at 298.15 K. Thermochim. Acta 406, 233–239 (2003)
Kell, G.S.: Density, thermal expansivity, and compressibility of liquid water from 0 °C to 150 °C: correlations and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale. J. Chem. Eng. Data 20, 97–105 (1975)
McMillan, W.G., Mayer, J.E.: The statistical thermodynamics of multicomponent systems. J. Chem. Phys. 13, 276–305 (1945)
Perron, G., Joly, D., Desnoyers, J.E.: Thermodynamics of the salting effect; free energies, enthalpies, entropies, heat capacities, and volumes of the ternary systems electrolyte–alcohol–water at 25 °C. Can. J. Chem. 56, 552–559 (1978)
Lu, Y., Cheng, Q., Chen, Y.: The enthalpic interaction parameters of acetamide with some alkali-metal chlorides in water at 298.15 K. Thermochim. Acta 334, 29–32 (1999)
Lu, Y., Han, Y., Liu, M.: Enthalpic and volumetric studies of the interactions of propionamide in aqueous carboxylic acid solutions at 298.15 K. Thermochim. Acta 416, 65–70 (2004)
Herskovits, T.T., Kelly, T.M.: Viscosity studies of aqueous solutions of alcohols, ureas, and amides. J. Phys. Chem. 77, 381–388 (1973)
Robinson, A.L.: The differential entropy of dilution in aqueous solutions of amino acids. J. Chem. Phys. 14, 588–590 (1946)
Hedwig, G.R., Lilley, T.H., Linsdell, H.: Calorimetric and volumetric studies of the interactions of same amides in water and in 6 mol dm−3 aqueous guanidinium chloride. J. Chem. Soc. Faraday Trans. 87, 2975–2982 (1991)
Visser, C., Perron, G., Desnoyers, J.E.: Volumes and heat capacities of ternary aqueous systems at 25 °C. Mixtures of urea, tert-butyl alcohol, dimethylformamide, and water. J. Am. Chem. Soc. 99, 5894–5900 (1977)
Matubyasi, N.: Matching-mismatching of water geometry and hydrophobic hydration. J. Am. Chem. Soc. 116, 1450–1456 (1994)
Scioxtino, F., Geiger, A., Stanley, H.E.: Network defects and molecular mobility in liquid water. J. Chem. Phys. 96, 3857–3865 (1992)
Gallardo, M.A., Lilley, T.H., Linsdell, H.: Aqueous solutions containing amino acids and peptides: Part 29. The enthalpies of dilution of some amino acids at 25 °C. Thermochim. Acta 233, 41–49 (1993)
Franks, F.: Hydrophobic interactions—a historical perspective. Faraday Symp. Chem. Soc. 17, 7–12 (1982)
Tanaka, H., Nakanishi, H., Toufionra, H.: Computer experiments on aqueous solutions. VI. Potential energy function for tert-butyl alcohol dimer and molecular dynamics calculation of 3 mol% aqueous solution of tert-butyl alcohol. J. Chem. Phys. 81, 4065–4073 (1984)
Lu, Y., Cheng, Q., Liu, M.: Calorimetric and volumetric studies of the interactions of butyramide in aqueous carboxylic acid solutions at 298.15 K. Thermochim. Acta 386, 103–110 (2002)
Frank, H.S., Wen, W.Y.: Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: a suggested picture of water structure. Discuss. Faraday Soc. 24, 133–140 (1957)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Tian, Y. & Lu, Y. Calorimetric and Volumetric Studies of the Interactions of Propionamide in Aqueous Alkan-1-ol Solutions at 298.15 K. J Solution Chem 37, 35–44 (2008). https://doi.org/10.1007/s10953-007-9221-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-007-9221-7