Abstract
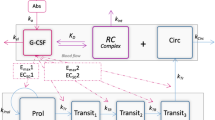
Neutropenia is a common side-effect of oncology drugs. We aimed to study the impact of exposure and dosing schedule on neutropenia to guide selection of dosing schedules that minimize neutropenia potential while maintaining the desired minimum concentration (Cmin) required for target engagement. Dose, frequency and PK parameters were chosen for five hypothetical drugs of various half-lives to (1) achieve same exposure with continuous dosing and evaluate impact of 4 intermittent dosing schedules; and (2) achieve same nadir for continuous and intermittent dosing and evaluate impact on % time above Cmin, a surrogate assumed to indicate target engagement. Absolute neutrophil count (ANC) profiles were simulated using Friberg model, a widely used semi-mechanistic myelosuppression model, assuming drug concentration directly reduce the proliferation rate of stem cells and progenitor cells in proliferation compartment. The correlations between different PK measures and neutropenia metrics were explored. In (1), when the same daily dose was used, intermittent schedules offered better management of ANC nadir. The reduced average drug exposure with intermittent dosing led to lower% time above Cmin. In (2), when the dose was adjusted to achieve the same nadir, drugs with moderate half-life (8–48 h) showed similar % time above Cmin regardless of schedule, while continuous dosing was better for a short half-life (4 h). Area under the concentration curve (AUC) was highly correlated with neutropenia. In summary, continuous dosing, with the dose selected correctly, is most effective to maintain % time above Cmin while providing similar tolerability as intermittent dosing with a higher dose. But dose interruptions could be required to manage individual toxicities. Intermittent schedules, on the other hand, allow recovery of ANC, enabling more orderly schedules.







Similar content being viewed by others
References
Dinan MA, Hirsch BR, Lyman GH (2015) Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Canc Netw 13(1):e1–7
Silber JH, Fridman M, DiPaola RS, Erder MH, Pauly MV, Fox KR (1998) First-cycle blood counts and subsequent neutropenia, dose reduction, or delay in early-stage breast cancer therapy. J Clin Oncol 16(7):2392–2400. https://doi.org/10.1200/JCO.1998.16.7.2392
Yoshida Y, Aisu N, Mogi A, Komono A, Sakamoto R, Kojima D, Mera T, Hasegawa S (2017) Difference in neutropenia due to administration schedule of TAS-102. Case Rep Oncol 10(1):226–229. https://doi.org/10.1159/000460242
Mangas-Sanjuan V, Buil-Bruna N, Garrido MJ, Soto E, Troconiz IF (2015) Semimechanistic cell-cycle type-based pharmacokinetic/pharmacodynamic model of chemotherapy-induced neutropenic effects of diflomotecan under different dosing schedules. J Pharmacol Exp Ther 354(1):55–64. https://doi.org/10.1124/jpet.115.223776
Lu D, Joshi A, Li H, Zhang N, Ren MM, Gao Y, Wada R, Jin JY (2014) Model-based meta-analysis for quantifying Paclitaxel dose response in cancer patients. CPT Pharmacometrics Syst Pharmacol 3:e115. https://doi.org/10.1038/psp.2014.14
Sun W, O’Dwyer PJ, Finn RS, Ruiz-Garcia A, Shapiro GI, Schwartz GK, DeMichele A, Wang D (2017) Characterization of neutropenia in advanced cancer patients following palbociclib treatment using a population pharmacokinetic-pharmacodynamic modeling and simulation approach. J Clin Pharmacol 57(9):1159–1173. https://doi.org/10.1002/jcph.902
Carpio C, Ysebaert L, Cordoba R, Santoro A, López-Martín JA, Sancho JM, Panizo C, Gharibo MM, Rasco DW, Stoppa A-M, Damian S, Wei X, Hagner P, Hege K, Carrancio S, Gandhi AK, Pourdehnad M, Ribrag V (2015) CC-122 Dosing on a novel intermittent schedule mitigates neutropenia and maintains clinical activity in subjects with relapsed or refractory diffuse large B cell lymphoma. Blood 126(23):1494–1494
Tolaney S, Forero-Torres A, Boni V, Bachelot T, Lu Y-S, Maur M, Fasolo A, Motta M, Pan C, Dobson J, Hewes B, Chin Lee S (2017) Abstract P4–22-12: Ribociclib + fulvestrant in postmenopausal women with HR+, HER2– advanced breast cancer (ABC). Cancer Res 77(4 Supplement):P4-22-12. https://doi.org/10.1158/1538-7445.sabcs16-p4-22-12
Sun J, Wei Q, Zhou Y, Wang J, Liu Q, Xu H (2017) A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol 11(Suppl 5):87. https://doi.org/10.1186/s12918-017-0464-7
Wu P, Nielsen TE, Clausen MH (2015) FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 36(7):422–439. https://doi.org/10.1016/j.tips.2015.04.005
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20(24):4713–4721. https://doi.org/10.1200/JCO.2002.02.140
Soto E, Keizer RJ, Troconiz IF, Huitema AD, Beijnen JH, Schellens JH, Wanders J, Cendros JM, Obach R, Peraire C, Friberg LE, Karlsson MO (2011) Predictive ability of a semi-mechanistic model for neutropenia in the development of novel anti-cancer agents: two case studies. Invest New Drugs 29(5):984–995. https://doi.org/10.1007/s10637-010-9437-z
Soto E, Staab A, Tillmann C, Trommeshauser D, Fritsch H, Munzert G, Troconiz IF (2010) Semi-mechanistic population pharmacokinetic/pharmacodynamic model for neutropenia following therapy with the Plk-1 inhibitor BI 2536 and its application in clinical development. Cancer Chemother Pharmacol 66(4):785–795. https://doi.org/10.1007/s00280-009-1223-2
Patel M, Palani S, Chakravarty A, Yang J, Shyu WC, Mettetal JT (2014) Dose schedule optimization and the pharmacokinetic driver of neutropenia. PLoS ONE 9(10):e109892. https://doi.org/10.1371/journal.pone.0109892
Sorbe B, Graflund M, Nygren L, Horvath G (2013) A study of docetaxel weekly or every three weeks in combination with carboplatin as first line chemotherapy in epithelial ovarian cancer: hematological and non-hematological toxicity profiles. Oncol Lett 5(4):1140–1148. https://doi.org/10.3892/ol.2013.1146
Zhu J, Liu R, Jiang Z, Wang P, Yao Y, Shen Z (2015) Optimization of drug regimen in chemotherapy based on semi-mechanistic model for myelosuppression. J Biomed Inform 57:20–27. https://doi.org/10.1016/j.jbi.2015.06.021
Cameron DA, Massie C, Kerr G, Leonard RC (2003) Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer 89(10):1837–1842. https://doi.org/10.1038/sj.bjc.6601366
Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi FV, Cigolari S, Manzione L, Illiano A, Barbera S, Robbiati SF, Frontini L, Piazza E, Ianniello GP, Veltri E, Castiglione F, Rosetti F, Gebbia V, Seymour L, Chiodini P, Perrone F (2005) Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol 6(9):669–677. https://doi.org/10.1016/S1470-2045(05)70255-2
Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C (1999) Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer 80(11):1763–1766. https://doi.org/10.1038/sj.bjc.6690594
Pierrillas PB, Fouliard S, Chenel M, Hooker AC, Friberg LF, Karlsson MO (2018) Model-based adaptive optimal design (MBAOD) improves combination dose finding designs: an example in oncology. AAPS J 20(2):39. https://doi.org/10.1208/s12248-018-0206-9
Pastor ML, Laffont CM, Gladieff L, Chatelut E, Concordet D (2015) Model-based approach to early predict prolonged high grade neutropenia in carboplatin-treated patients and guide G-CSF prophylactic treatment. Pharm Res 32(2):654–664. https://doi.org/10.1007/s11095-014-1493-1
Everds NE, Tarrant JM (2013) Unexpected hematologic effects of biotherapeutics in nonclinical species and in humans. Toxicol Pathol 41(2):280–302. https://doi.org/10.1177/0192623312467400
Bender BC, Schaedeli-Stark F, Koch R, Joshi A, Chu YW, Rugo H, Krop IE, Girish S, Friberg LE, Gupta M (2012) A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol 70(4):591–601. https://doi.org/10.1007/s00280-012-1934-7
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
YG, NHB, HX, and DO wrote the manuscript. YG, NHB, HX, and DO designed the research. YG, NHB, and DO performed the research. YG, NHB, and DO analyzed data.
Corresponding author
Ethics declarations
Conflict of interest
All authors were employees of Janssen Research & Development at the time of this study. No other potential conflicts of interest were disclosed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Y., Haddish-Berhane, N., Xie, H. et al. Optimization of clinical dosing schedule to manage neutropenia: learnings from semi-mechanistic modeling simulation approach. J Pharmacokinet Pharmacodyn 47, 47–58 (2020). https://doi.org/10.1007/s10928-019-09667-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-019-09667-y