Abstract
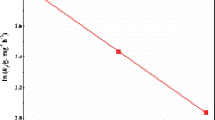
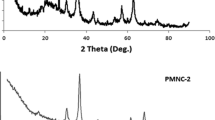
The purpose of this research was to fabricate starch based magnetic nanobiocomposites using two different starch types (corn and wheat) and to examine the effects of some experimental parameters (such as starch concentration, NaOH concentration and aging time of nanoparticles) that play a role in the synthesis products. The characterization of the starch-based magnetic-nanobiocomposite materials were realized with several techniques. In the previous study, MW_S3 and MC_S3 from starch stabilized magnetite nanobiocomposites were selected for further studies with smaller particle size and large surface areas, as a result of calculations based on XRD results and BET surface area. The usability of starch-based magnetic-nanobiocomposite materials, which can provide rapid separation with their magnetic properties and are not toxic, in removing Sr(II) ions from aqueous solutions has been investigated. The parameters affecting the biosorption were investigated using a full factorial experimental design. In the biosorption study, pH, temperature, initial Sr(II) concentration and contact time were determined as four independent variables. The regression coefficients were found using the least squares method and the response surface graphs were created according to the polynomial equation obtained from the full factorial experimental design. ANOVA (analysis of variance) analysis within the 95% confidence interval of the model applied for the full factorial experimental design was examined and the compatibility of the model with the experimental findings was examine. It is seen that the biosorption of Sr(II) on MW_S3 and MC_S3 nanobiocomposites increases with increasing concentration in the range of 25–75 ppm. As a result of the regression analysis, it was observed that pH was statistically significant (p < 0.05) and had an increasing effect for MW_S3. Evaluating the results obtained, it was found that the combined effects of the parameters on the biosorption of Sr(II) on MW_S3 adsorbent were not significant, but the combined effects of concentration and time were only significant on the adsorption of Sr(II) on MC_S3 adsorbent. From the solution of the equation obtained in the full factorial experimental design, it has been determined that the optimum biosorption conditions for MW_S3 adsorbent are; pH is 7, temperature is 34.87 °C, initial Sr(II) concentration is 75 mg/L and contact time is 30 min. Optimum adsorption conditions for MC_S3 adsorbent were determined to be pH is 8, temperature is 34.46 °C, initial Sr(II) concentration is 74.83 mg/L and contact time is 59.54 min. The composition and chemical state of the magnetic nanobiocomposites were investigated by XPS analysis after Sr(II) biosorption. For the purpose of determine the adsorption model, the relevant parameters were calculated using Langmuir, Freundlich and Dubinin–Radushkevich isotherms. Gibbs free energy change, enthalpy and entropy values, which are the values of adsorption thermodynamics, were calculated.














Similar content being viewed by others
References
Shubair T, Eljamal O, Tahara A, Sugihara Y, Matsunaga N (2019) Preparation of new magnetic zeolite nanocomposites for removal of strontium from polluted waters. J Mol Liq 288:111026
Ambashta RD, Sillanpää M (2010) Water purification using magnetic assistance: a review. J Hazard Mater 180:38
Faghihian H, Iravani M, Moayed M, Ghannadi-Maragheh M (2013) Preparation of a novel PAN–zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Chem Eng J 222:41
Bayramoglu G, Arica MY (2019) Star type polymer grafted and polyamidoxime modified silica coated-magnetic particles for adsorption of U (VI) ions from solution. Chem Eng Res Des 147:146
Vipin AK, Ling S, Fugetsu B (2016) Removal of Cs+ and Sr2+ from water using MWCNT reinforced zeolite-A beads. Microporous Mesoporous Mater 224:84
Oral AE, Aytas S, Yusan S, Sert S, Gok C, Elmastas Gultekin O (2020) Preparation and characterization of a graphene-based magnetic nanocomposite for the adsorption of lanthanum ions from aqueous solution. Anal Lett 53:1812
Karmaker SC, Eljamal O, Saha BB (2021) Response surface methodology for strontium removal process optimization from contaminated water using zeolite nanocomposites. Environ Sci Pollut Res 28:56535
Guévar C, Hertz A, Brackx E, Barré Y, Grandjean A (2017) Mechanisms of strontium removal by a Ba-titanate material for the wastewater treatment. J Environ Chem Eng 5:4948
Asl SH, Ahmadi M, Ghiasvand M, Tardast A, Katal R (2013) Artificial neural network (ANN) approach for modeling of Cr (VI) adsorption from aqueous solution by zeolite prepared from raw fly ash (ZFA). J Ind Eng Chem 19:1044
Paudyal H, Pangeni B, Inoue K, Ohto K, Kawakita H, Ghimire KN, Harada H, Alam S (2014) Adsorptive removal of strontium from water by using chemically modified orange juice residue. Sep Sci Technol 49:1244
Moghadam NH, Salehzadeh S, Rakhtshah J, Tanzadehpanah H, Moghadam AH, Hajibabaei F, Sharifinia S, Asl SS, Saidijam M (2018) Improving antiproliferative effect of the nevirapine on Hela cells by loading onto chitosan coated magnetic nanoparticles as a fully biocompatible nano drug carrier. Int J Biol Macromol 118:1220–1228
Saboktakin MR, Maharramov A, Ramazanov MA (2009) Synthesis and characterization of superparamagnetic nanoparticles coated with carboxymethyl starch (CMS) for magnetic resonance imaging technique. Carbohydr Polym 78:2
Low LE, Tan LT-H, Goh B-H, Tey BT, Ong BH, Tang SY (2019) Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int J Biol Macromol 127:76–84
Jia H, Song X-R, Liu S, Xu X, Zheng X-C, Peng Z-K, Liu P (2019) Assembly and superior performance of palladium nano-catalysts anchored to a magnetic konjac glucomannan-graphene oxide hybrid for H2 generation from ammonia borane. J Taiwan Inst Chem Eng 100:137–143
Almeida EA, Bellettini IC, Garcia FP, Farinacio MT, Nakamura CV, Rubira AF, Martins AF, Muniz EC (2017) Curcumin-loaded dual pH-and thermoresponsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr Polym 171:259–266
Mallakpour S, Nezamzadeh Ezhieh A (2018) Preparation and characterization of starch nanocomposite embedded with functionalized MWCNT: investigation of optical, morphological, thermal, and copper ions adsorption properties. Adv Polym Technol 37:2195
Özbay N, Yargıç AŞ, Yarbay-Şahin RZ, Önal E (2013) Full factorial experimental design analysis of reactive dye removal by carbon adsorption. J Chem 2013:1–13
Prill B, Yusan S (2021) Synthesis and characterization of magnetic nanoparticles functionalized with different starch types. Part Sci Technol 40:541–530
Elhalil A, Tounsadi H, Elmoubarki R, Mahjoubi FZ, Farnane M, Sadiq M, Abdennouri M, Qourzal S, Barka N (2016) Factorial experimental design for the optimization of catalytic degradation of malachite green dye in aqueous solution by fenton process. Water Resour Ind 15:41
Brasil JL, Martins LC, Ev RR, Dupont J, Dias SLP, Sales JAA, Airoldi C, Lima ÉC (2005) Factorial design for optimization of flow-injection preconcentration procedure for copper (II) determination in natural waters, using 2-aminomethylpyridine grafted silica gel as adsorbent and spectrophotometric detection. Int J Environ Anal Chem 85:475
Gürkan EH, Tibet Y, Çoruh S (2021) Application of full factorial design method for optimization of heavy metal release from lead smelting slag. Sustainability 13:4890
Garg UK, Kaur MP, Garg VK, Sud D (2008) Removal of nickel (II) from aqueous solution by adsorption on agricultural waste biomass using a response surface methodological approach. Bioresour Technol 99:1325
Can MY, Kaya Y, Algur OF (2006) Response surface optimization of the removal of nickel from aqueous solution by cone biomass of Pinus sylvestris. Bioresour Technol 97:1761
Okumuş ZÇ, Doğan TH, Temur H (2019) Removal of water by using cationic resin during biodiesel purification. Renew Energy 143:47
Akbas YA, Yusan S (2020) Development and characterization of non-treated and chemically modified olive pomace biosorbents to remove Ce(III) ions from aqueous solutions. J Radioanal Nucl Chem 323:763–772
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:1–11
Chakraborty S, Chowdhury S, Saha P (2011) Das: adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym 86:1533
Yusan S, Bampaiti A, Erenturk S, Noli F, Aslani MAA, Aytas S (2016) Sorption of Th(IV) onto ZnO nanoparticles and diatomite-supported ZnO nanocomposite: kinetics, mechanism and activation parameters. Radiochim Acta 104:635–647
Jaycock, MJ., Parfitt, GD (1981) Chemistry of interfaces. Ellis Horwood Limited Publishers, Chichester
Yusan S, Gok C, Erenturk S, Aytas S (2012) Adsorptive removal of thorium (IV) using calcined and flux calcined diatomite from Turkey: evaluation of equilibrium, kinetic and thermodynamic data. Appl Clay Sci 67–68:106–116
Yu Y, Zhuang Y-Y, Wang Z-H (2001) Adsorption of water-soluble dye onto functionalized resin. J Colloid Interface Sci 242:288
Bondar YuV, Alekseev SA (2020) Synthesis and evaluation of manganese dioxide with layered structure as an adsorbent for selective removal of strontium ions from aqueous solution. SN Appl Sci 2:1379
Funding
This study was financially supported by Ege University Scientific Research Project Unit Project No. FYL-2020-22227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prill, B., Sedir, U., Yusan, S. et al. Strontium (II) Biosorption Studies on Starch-Functionalized Magnetic Nanobiocomposites Using Full Factorial Design Method. J Polym Environ 30, 5148–5162 (2022). https://doi.org/10.1007/s10924-022-02575-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02575-2