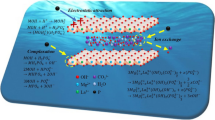
Abstract
Herein, we report the preparation of Fe(III) complexed polydopamine modified Mg/Al layered double hydroxides composite material (LDHs@PDA-Fe(III)) and its application to the removal of Cr(VI) in aqueous solution. LDHs@PDA-Fe(III) was characterized and analyzed by field-emission scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM–EDS), Fourier transformed infrared (FTIR), X-ray diffraction (XRD), X-ray photoelectron (XPS). The adsorption performance was studied through a series of adsorption experiments. Investigate the effects of pH, time, temperature, concentration and other factors. When C0 = 800 mg/L, T = 308 k and pH 3, the maximum adsorption capacity obtained in the experiment was 683.4 mg/g. In addition, after 5 adsorption cycles, LDHs@PDA-Fe(III) still shows excellent adsorption capacity and stability. Combining adsorption experiments and characterization analysis, it is inferred that the adsorption of Cr(VI) by LDHs@PDA-Fe(III) is the result of the synergistic effect of multiple adsorption mechanisms. Therefore, the efficient removal capacity and excellent stability make LDHs@PDA-Fe(III) an ideal adsorbent for removing Cr(VI) from aqueous solutions.










Similar content being viewed by others
References
Li Y, Zhu H, Zhang C, Cheng M, He H (2018) PEI-grafted magnetic cellulose for Cr(VI) removal from aqueous solution. Cellulose 25:4757–4769. https://doi.org/10.1007/s10570-018-1868-2
Chen H, Zhang Z, Zhong X, Zhuo Z, Tian S, Fu S, Chen Y, Liu Y (2021) Constructing MoS2/Lignin-derived carbon nanocomposites for highly efficient removal of Cr(VI) from aqueous environment. J Hazard Mater 408:124847. https://doi.org/10.1016/j.jhazmat.2020.124847
Bao S, Yang W, Wang Y, Yu Y, Sun Y, Li K (2020) PEI grafted amino-functionalized graphene oxide nanosheets for ultrafast and high selectivity removal of Cr(VI) from aqueous solutions by adsorption combined with reduction: Behaviors and mechanisms. Chem Eng J 399:125762. https://doi.org/10.1016/j.cej.2020.125762
Kretschmer I, Senn AM, Meichtry JM, Custo G, Halac EB, Dillert R, Bahnemann DW, Litter MI (2019) Photocatalytic reduction of Cr(VI) on hematite nanoparticles in the presence of oxalate and citrate. Appl Catal B 242:218–226. https://doi.org/10.1016/j.apcatb.2018.09.059
Bandehali S, Parvizian F, Moghadassi AR, Hosseini SM, Shen JN (2020) Fabrication of thin film-PEI nanofiltration membrane with promoted separation performances: Cr, Pb and Cu ions removal from water. J Polym Res. https://doi.org/10.1007/s10965-020-02056-x
Liang M, Ding Y, Zhang Q, Wang D, Li H, Lu L (2020) Removal of aqueous Cr(VI) by magnetic biochar derived from bagasse. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-78142-3
Shi Y, Shan R, Lu L, Yuan H, Jiang H, Zhang Y, Chen Y (2020) High-efficiency removal of Cr(VI) by modified biochar derived from glue residue. J Clean Prod 254:119935. https://doi.org/10.1016/j.jclepro.2019.119935
Zhu K, Gao Y, Tan X, Chen C (2016) Polyaniline-modified Mg/Al layered double hydroxide composites and their application in efficient removal of Cr(VI). ACS Sustain Chem Eng 4:4361–4369. https://doi.org/10.1021/acssuschemeng.6b00922
Yu XY, Luo T, Jia Y, Xu RX, Gao C, Zhang YX, Liu JH, Huang XJ (2012) Three-dimensional hierarchical flower-like Mg-Al-layered double hydroxides: highly efficient adsorbents for As(v) and Cr(vi) removal. Nanoscale 4:3466–3474. https://doi.org/10.1039/c2nr30457k
Luo M, Huang C, Chen F, Chen C, Li H (2020) Removal of aqueous Cr(VI) using magnetic-gelatin supported on Brassica-straw biochar. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2020.1785889
Lu X, Liu X, Zhang W, Wang X, Wang S, Xia T (2019) The residue from the acidic concentrated lithium bromide treated crop residue as biochar to remove Cr (VI). Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.122348
Guo J, Li JJ, Wang CC (2019) Adsorptive removal of Cr(VI) from simulated wastewater in MOF BUC-17 ultrafine powder. J Environ Chem Eng 7:102909. https://doi.org/10.1016/j.jece.2019.102909
Li LL, Feng XQ, Han RP, Zang SQ, Yang G (2017) Cr(VI) removal via anion exchange on a silver-triazolate MOF. J Hazard Mater 321:622–628. https://doi.org/10.1016/j.jhazmat.2016.09.029
Hu B, Ai Y, Jin J, Hayat T, Alsaedi A, Zhuang L, Wang X (2020) Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2:47–64. https://doi.org/10.1007/s42773-020-00044-4
Zhang W, Qian L, Chen Y, Ouyang D, Han L, Shang X, Li J, Gu M, Chen M (2021) Nanoscale zero-valent iron supported by attapulgite produced at different acid modification: Synthesis mechanism and the role of silicon on Cr(VI) removal. Chemosphere 267:129183. https://doi.org/10.1016/j.chemosphere.2020.129183
Goh KH, Lim TT, Dong Z (2008) Application of layered double hydroxides for removal of oxyanions: A review. Water Res 42:1343–1368. https://doi.org/10.1016/j.watres.2007.10.043
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. Innov 2:100076. https://doi.org/10.1016/j.xinn.2021.100076
Huang D, Liu C, Zhang C, Deng R, Wang R, Xue W, Luo H, Zeng G, Zhang Q, Guo X (2019) Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour Technol 276:127–132. https://doi.org/10.1016/j.biortech.2018.12.114
Xu H, Zhu S, Xia M, Wang F (2021) Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@PANI@LDH composite: experimental and adsorption mechanism study. J Hazard Mater 402:123815. https://doi.org/10.1016/j.jhazmat.2020.123815
Zhu Y, He X, Xu J, Fu Z, Wu S, Ni J, Hu B (2021) Insight into efficient removal of Cr(VI) by magnetite immobilized with Lysinibacillus sp. JLT12: Mechanism and performance. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127901
Wang Q, Lin Q, Li Q, Li K, Wu L, Li S, Liu H (2021) As(III) removal from wastewater and direct stabilization by in-situ formation of Zn-Fe layered double hydroxides. J Hazard Mater 403:123920. https://doi.org/10.1016/j.jhazmat.2020.123920
Liang L, Xi F, Tan W, Meng X, Hu B, Wang X (2021) Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 3:255–281. https://doi.org/10.1007/s42773-021-00101-6
Li J, Chen S, Xiao H, Yao G, Gu Y, Yang Q, Yan B (2020) Highly efficient removal of Cr(vi) ions from wastewater by the pomegranate-like magnetic hybrid nano-adsorbent of polydopamine and Fe 3 O 4 nanoparticles. New J Chem 44:12785–12792. https://doi.org/10.1039/d0nj01293a
Lv X, Qin X, Wang K, Peng Y, Wang P, Jiang G (2019) Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: enhanced performance in Cr(VI) removal, mechanism and regeneration. J Hazard Mater 373:176–186. https://doi.org/10.1016/j.jhazmat.2019.03.091
Furtado LM, Ando RA, Petri DFS (2020) Polydopamine-coated cellulose acetate butyrate microbeads for caffeine removal. J Mater Sci 55:3243–3258. https://doi.org/10.1007/s10853-019-04169-1
Kallem P, Othman I, Ouda M, Hasan SW, AlNashef I, Banat F (2021) Polyethersulfone hybrid ultrafiltration membranes fabricated with polydopamine modified ZnFe2O4 nanocomposites: applications in humic acid removal and oil/water emulsion separation. Process Saf Environ Prot 148:813–824. https://doi.org/10.1016/j.psep.2021.02.002
Yang HC, Wu QY, Wan LS, Xu ZK (2013) Polydopamine gradients by oxygen diffusion controlled autoxidation. Chem Commun 49:10522–10524. https://doi.org/10.1039/c3cc46127k
Zhang Q, Li Y, Yang Q, Chen H, Chen X, Jiao T, Peng Q (2018) Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J Hazard Mater 342:732–740. https://doi.org/10.1016/j.jhazmat.2017.08.061
Zhang C, Ou Y, Lei WX, Wan LS, Ji J, Xu ZK (2016) CuSO4/H2O2-induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew Chem Int Ed 55:3054–3057. https://doi.org/10.1002/anie.201510724
Zhang L, Shi J, Jiang Z, Jiang Y, Meng R, Zhu Y, Liang Y, Zheng Y (2011) Facile preparation of robust microcapsules by manipulating metal-coordination interaction between biomineral layer and bioadhesive layer. ACS Appl Mater Interfaces 3:597–605. https://doi.org/10.1021/am101184h
Zhou D, Yang L, Yu L, Kong J, Yao X, Liu W, Xu Z, Lu X (2015) Fe/N/C hollow nanospheres by Fe(iii)-dopamine complexation-assisted one-pot doping as nonprecious-metal electrocatalysts for oxygen reduction. Nanoscale 7:1501–1509. https://doi.org/10.1039/c4nr06366j
Wilker JJ (2010) The iron-fortified adhesive system of marine mussels. Angew Chem Int Ed 49:8076–8078. https://doi.org/10.1002/anie.201003171
Chen B, Cao Y, Zhao H, Long F, Feng X, Li J, Pan X (2020) A novel Fe3+-stabilized magnetic polydopamine composite for enhanced selective adsorption and separation of Methylene blue from complex wastewater. J Hazard Mater 392:122263. https://doi.org/10.1016/j.jhazmat.2020.122263
Yu Z, Zhang X, Huang Y (2013) Magnetic chitosan-iron(III) hydrogel as a fast and reusable adsorbent for chromium(VI) removal. Ind Eng Chem Res 52:11956–11966. https://doi.org/10.1021/ie400781n
Wen T, Wu X, Tan X, Wang X, Xu A (2013) One-pot synthesis of water-swellable Mg-Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions. ACS Appl Mater Interfaces 5:3304–3311. https://doi.org/10.1021/am4003556
Zhu K, Chen C, Xu H, Gao Y, Tan X, Alsaedi A, Hayat T (2017) Cr(VI) reduction and immobilization by core-double-shell structured magnetic Polydopamine@Zeolitic Idazolate frameworks-8 microspheres. ACS Sustain Chem Eng 5:6795–6802. https://doi.org/10.1021/acssuschemeng.7b01036
Nematollahzadeh A, Seraj S, Mirzayi B (2015) Catecholamine coated maghemite nanoparticles for the environmental remediation: hexavalent chromium ions removal. Chem Eng J 277:21–29. https://doi.org/10.1016/j.cej.2015.04.135
Zhu J, Tsehaye MT, Wang J, Uliana A, Tian M, Yuan S, Li J, Zhang Y, Volodin A, Van der Bruggen B (2018) A rapid deposition of polydopamine coatings induced by iron (III) chloride/hydrogen peroxide for loose nanofiltration. J Colloid Interface Sci 523:86–97. https://doi.org/10.1016/j.jcis.2018.03.072
Zhao S, Zhan Y, Wan X, He S, Yang X, Hu J, Zhang G (2020) Selective and efficient adsorption of anionic dyes by core/shell magnetic MWCNTs nano-hybrid constructed through facial polydopamine tailored graft polymerization: Insight of adsorption mechanism, kinetic, isotherm and thermodynamic study. J Mol Liq 319:114289. https://doi.org/10.1016/j.molliq.2020.114289
Shi P, Hu X, Duan M (2021) A UIO-66/tannic acid/chitosan/polyethersulfone hybrid membrane-like adsorbent for the dynamic removal of dye and Cr (VI) from water. J Clean Prod 290:125794. https://doi.org/10.1016/j.jclepro.2021.125794
Liu P, Wang X, Ma J, Liu H, Ning P (2019) Highly efficient immobilization of NZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere 220:1003–1013. https://doi.org/10.1016/j.chemosphere.2018.12.163
Matusik J, Hyla J, Maziarz P, Rybka K, Leiviskä T (2019) Performance of halloysite-Mg/Al LDH materials for aqueous as(V) and Cr(VI) removal. Materials (Basel) 12:1–16. https://doi.org/10.3390/ma12213569
Zhang Y, Li M, Li J, Yang Y, Liu X (2019) Surface modified leaves with high efficiency for the removal of aqueous Cr (VI). Appl Surf Sci 484:189–196. https://doi.org/10.1016/j.apsusc.2019.04.088
Ma L, Shi X, Zhang X, Dong S, Li L (2019) Electrospun cellulose acetate–polycaprolactone/chitosan core-shell nanofibers for the removal of Cr(VI). Phys Status Solidi Appl Mater Sci 216:1–9. https://doi.org/10.1002/pssa.201900379
Lyu J, Zhang N, Liu H, Zeng Z, Zhang J, Bai P, Guo X (2017) Adsorptive removal of boron by zeolitic imidazolate framework: kinetics, isotherms, thermodynamics, mechanism and recycling. Sep Purif Technol 187:67–75. https://doi.org/10.1016/j.seppur.2017.05.059
Kwak HW, Lee H, Lee KH (2020) Surface-modified spherical lignin particles with superior Cr(VI) removal efficiency. Chemosphere 239:124733. https://doi.org/10.1016/j.chemosphere.2019.124733
Wang J, Wang P, Wang H, Dong J, Chen W, Wang X, Wang S, Hayat T, Alsaedi A, Wang X (2017) Preparation of molybdenum disulfide coated Mg/Al layered double hydroxide composites for efficient removal of chromium(VI). ACS Sustain Chem Eng 5:7165–7174. https://doi.org/10.1021/acssuschemeng.7b01347
Sun Y, Yu F, Li L, Ma J (2021) Adsorption-reduction synergistic effect for rapid removal of Cr (VI) ions on superelastic NH2-graphene sponge. Chem Eng J. https://doi.org/10.1016/j.cej.2021.129933
Dong L, Deng R, Xiao H, Chen F, Zhou Y, Li J, Chen S, Yan B (2019) Hierarchical polydopamine coated cellulose nanocrystal microstructures as efficient nanoadsorbents for removal of Cr(VI) ions. Cellulose 26:6401–6414. https://doi.org/10.1007/s10570-019-02529-3
Wang H, Guo H, Zhang N, Chen Z, Hu B, Wang X (2019) Enhanced photoreduction of U(VI) on C3N4 by Cr(VI) and bisphenol A: ESR, XPS, and EXAFS investigation. Environ Sci Technol 53:6454–6461. https://doi.org/10.1021/acs.est.8b06913
Liu F, Hua S, Wang C, Qiu M, Jin L, Hu B (2021) Adsorption and reduction of Cr(VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 279:130539. https://doi.org/10.1016/j.chemosphere.2021.130539
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Luo, M., Chen, F. et al. Fe(III) Complexed Polydopamine Modified Mg/Al Layered Double Hydroxide Enhances the Removal of Cr(VI) from Aqueous Solutions. J Polym Environ 30, 2547–2558 (2022). https://doi.org/10.1007/s10924-022-02375-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02375-8