Abstract
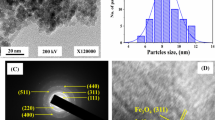
A new high-swelling multi-component superabsorbent composite Na-CMC-g-P(AMPS-co-AA-co-AM)/NanoFe3O4 (SANCHs) was successfully designed and utilized for effective removal of 134Cs, 85Sr and 60Co radionuclides from radioactive wastewater. The structure and morphology of the superabsorbent composite have been mapped and characterized using different techniques (SEM, XRD, TEM, FTIR, and TGA). Superabsorbent hydrogel composite networks with magnetic iron particles have been disclosed to provide an advantage over previous methods. By using distinct models for kinetic investigation, mathematical fitting was observed to be pseudo-second-order with (R2) 0.987, 0.999, and 0.998 respectively for 134Cs, 60Co, and 85Sr. In addition, the highest adsorption capability was found to be 23.9, 43, and 47.2 mg g−1 respectively. The variation in the magnitude of enthalpy (ΔH) and free energy (ΔG) verify the spontaneous state of the adsorption reaction suggesting an endothermic process. Such a functionalized magnetic nanocomposite adsorbent demonstrates excellent prospective applications for effective separation of certain radionuclides from radioactive liquid waste.















Similar content being viewed by others
References
Ngwenya N, Chirwa EMN (2010) Single and binary component sorption of the fission products Sr2+, Cs+ and Co2+ from aqueous solutions onto sulphate reducing bacteria. Miner Eng 23:463–470
Camacho LM, Deng SG, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175:393–398
Li XY, Cui YH, Feng YJ, Xie ZM, Gu JD (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res 39:1972–1981
El-Naas MH, Al-Muhtaseb SA, Makhlouf S (2009) Biodegradation of phenol by Pseudomonas putida immobilized in polyvinylalcohol (PVA) gel. J Hazard Mater 164:720–725
Hamed MM, Shahr El-Din AM, Abdel-Galil EA (2019) Nanocomposite of polyaniline functionalized tafla: synthesis, characterization, and application as a novel sorbent for selective removal of Fe(III). J Radioanal Nucl Chem 322:663–676
Al-Aidy H, Amdeha E (2020) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: the new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ An Chem. https://doi.org/10.1080/03067319.2020.1711896
Sharma S, Dua A, Malik A (2014) Poly aspartic acid based superabsorbent polymers. Euro Polym J 59:363–376
Zhang XY, Wang XP, Li L, Zhang SS, Wu RN (2015) Preparation and swelling behaviors of a high temperature resistant superabsorbent using tetraallylammonium chloride as crosslinking agent. React Funct Polym 87:15–21
Mu YH, Du DY, Yang RC, Xu ZW (2015) Preparation and performance of poly (acrylic acid– methacrylic acid)/montmorillonite microporous superabsorbent nanocomposite. Mater Lett 142:94–96
Kosemund K, Schlatter H, Ochsenhirt JL, Krause EL, Marsman DS, Erasala GN (2009) Safety evaluation of superabsorbent baby diapers. Regul Toxicol Pharm 53:81–89
Wang Q, Zhang JP, Wang AQ (2009) Preparation and characterization of a novel pH-sensitive chitosan-g-poly(acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr Polym 78:731–737
Bhattacharyya R, Ray SK (2014) Micro- and nano-sized bentonite filled composite superabsorbents of chitosan and acrylic copolymer for removal of synthetic dyes from water. Appl Clay Sci 101:510–520
Yang F, Li G, He YG, Ren FX, Wang GX (2009) Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym 78:95–99
Suo AL, Qian JM, Yao Y, Zhang WG (2007) Synthesis and properties of carboxymethyl cellulose-graft-poly(acrylic acidco- acrylamide) as anovel cellulose-based superabsorbent. J Appl Polym Sci 103:1382–1388
Dias AMGC, Hussain A, Marcos AS, Roque ACA (2011) A biotechnological perspective on the application of ironoxide magnetic colloids modified with polysaccharides. Biotechnol Adv 29:142–155
Farag RK, El-saied HA (2019) Green modification of waste cotton surface fabric by (2-acrylamide-2-methyl propane sulfonic acid, AMPS) and nano magnetite particles. Egypt J Pet. https://doi.org/10.21608/ejchem.2019.12017.1759
Raslan HA, El-Saied HA, Mohamed RM, Yousif NM (2019) Gamma radiation induced fabrication of styrene butadiene rubber/ magnetite nanocomposites for positive temperature coefficient thermistors application. Compos Part B 176:107326
Ghasemzadeh H, Ghanaat F (2014) Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis and characterization. J Polym Res 21:355–368
Rashidzadeh A, Olad A, Salari D, Reyhanitabar A (2014) On the preparation and swelling properties of hydrogel nanocomposite based on sodium alginate-g-poly(acrylic acid-co-acrylamide)/clinoptilolite and its application as slow release fertilizer. J Polym Res 21:344–359
Yadollahi M, Namazi H, Aghazadeh M (2015) Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int J Biol Macromol 79:269–277
Yan H, Zhang W, Kan X, Dong L, Jiang Z, Li H, Yang H, Cheng R (2011) Sorption of methylene blue by carboxymethyl cellulose and reuse process in a secondary sorption. Colloids Surf A 380:143–151
Jia Z, Wang Q, Liu J, Xu J, Zhu R (2013) Effective removal of phosphate from aqueous solution using mesoporous rod like NiFe2O4 as magnetically separable ad-sorbent. Colloids Surf A 436:495–503
Lim DW, Yoon KJ, Ko SW (2000) Synthesis of AA-based superabsorbent interpenetrated with sodium PVA sulfate. J Appl Polym Sci 78(14):2525–2532
Poorna KSVC, Singh A, Rathore A, Kumar A (2016) Novel cross linked guar gum-g-poly(acrylate) porous superabsorbent hydrogels: characterization and swelling behaviour in different environments. Carbohydr Polym 149:175–185
Yadav M, Rhee KY, Park SJ (2014) Synthesis and characterization of grapheme oxide/carboxymethylcellulose/alginate composite blend films. Carbohydr Polym 110:18–25
Mondal MIH, Yeasmin MS, Rahman MS (2015) Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int J Biol Macromol 79:144–150
Gnanaprakash G, Philip J, Jayakumar T, Raj B (2007) Effect of digestion time and alkaliaddition rate on physical properties of magnetite nanoparticles. J Phys Chem 111:7978–7986
Tingaut P, Zimmermann T, Lopez-Suevos F (2009) Synthesis and characterization of bionanocomposites with tunable properties from poly (lactic acid) and acetylated microfibrillated cellulose. Biomacromolcules 11:454–464
Shen J, Yan B, Li T, Long Y, Li N, Ye M (2012) Study on graphene- oxide-based polyacrylamide composite hydrogels. Compos A 43(9):1476–1481
Ge H, Wang S (2014) Thermal preparation of chitosan-acrylic acid superabsorbent: optimization, characteristic and water absorbency. Carbohydr Polym 113:296–303
Işıklan N, Küçükbalcı G (2012) Microwave-induced synthesis of alginate-graft-poly(N-isopropylacrylamide) and drug release properties of dual pH- and temperature-responsive beads. Eur J Pharmacol Biopharm 82:316–331
Tally M, Atassi Y (2016) Synthesis and characterization of pH-sensitive superabsorbent hydrogels based on sodium alginate-g-poly(acrylic acid-co-acrylamide) obtained via an anionic surfactant micelle templating under microwave irradiation. Polym Bull 22(9):1–26
Gils PS, Ray D, Mohanta GP, Manavalan R, Sahoo PK (2009) Designing of new acrylic based macroporous superabsorbent polymer hydrogel and its suitability for drug delivery. Int J Pharm Pharm Sci 1:43–54
Hosseinzadeh H, Sadeghzadeh M, Badazadeh M (2011) Preparation and properties of carrageenan-gpoly (acrylic acid)/bentonite superabsorbent composite. J Boimater Nanobiotechnol 2:311–317
Li P, Kim NH, Siddaramaiah JH (2009) Swelling behavior of polyacrylamide/laponite clay nanocomposite hydrogels: pH-sensitive property. Compos Part B 40:275–283
Lanthong P, Nuisin R, Kiatkamjornwong S (2006) Graft copolymerization, characterization, and degradation of cassava starch-gacrylamide/ itaconic acid superabsorbents. Carbohydr Polym 66:229–245
El-Kafrawy AF, El-Saeed SM, Farag RK, El-Saied HA, Abdel-Raouf ME (2017) Adsorbents based on natural polymers for removal of some heavy metals from aqueous solution. Egypt J Petrol 26(1):23–32
Shahr El-Din AM, Monir T, Sayed MA (2019) Nano-sized Prussian blue immobilized costless agro-industrial waste for the removal of cesium-137 ions. Environ Sci Pollut Res 26(25):25550–25563
Alamudy HA, Cho K (2018) Selective adsorption of cesium from an aqueous solution by a montmorillonite-prussian blue hybrid. Chem Eng J 349:595–602
Labib Sh, Shahr El-Din AM, Allan KF, Attallah MF (2020) Synthesis of highly deficient nano SrCoOx for the purification of lanthanides from monazite concentrate. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-020-07031-w
Abdel-Galil EA, Moloukhia H, Abdel-Khalik M, Mahrous SS (2018) Synthesis and physico-chemical characterization of cellulose/HO7Sb3 nanocomposite as adsorbent for the removal of some radionuclides from aqueous solutions. Appl Radiat Isotopes 140:363–373
Chen JH, Li GP, Liu QL, Ni JC, Wu WB, Lin JM (2010) Cr(III) ionic imprinted polyvinyl alcohol/sodium alginate (PVA/SA) porous composite membranes for selective adsorption of Cr(III) ions. Chem Eng J 165:465–473
Ali SA, Al Hamouz OC, Hassan NM (2013) Novel cross-linked polymers having pH-responsive amino acid residues for the removal of Cu2+ from aqueous solution at low concentrations. J Hazard Mater 248–249:47–58
Hamed MM, Aly MI, Nayl AA (2016) Kinetics and thermodynamics studies of cobalt, strontium and cesium sorption on marble from aqueous solution. Chem Ecol 32:68–87
Mansy MS, Hassan RS, Selim YT, Kenawy SH (2017) Evaluation of synthetic aluminum silicate modified by magnesia for the removal of 137Cs, 60Co and 152+154Eu from low-level radioactive waste. Appl Radiat Isotopes 130:198–205
Attallah MF, Abd-Elhamid AI, Ahmed IM, Aly HF (2018) Possible use of synthesized nano silica functionalized by Prussian blue as sorbent for removal of certain radionuclides from liquid radioactive waste. J Mol Liq 261:379–386
Li T, He F, Dai YD (2016) Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J Radioanal Nucl Chem 310:1139–1145
Bouzaida I, Ferronato CJM, Rammah ME, Hermann JM (2004) Photocatalytic degradation of the anthraquinonic dye, acid blue (AB25): a kinetic approach. J Photochem Photobiol A 168:23–30
Rivas B, Schiappacasse N (2003) Poly(acrylic acid-co-vinylsulfonic acid): synthesis, characterization, and properties as polychelatogen. J Appl Polym Sci 88:1698–1704
Acknowledgements
Authors are thankful to the Petroleum Research Institute, and Atomic Energy Authority, Cairo, Egypt for the fruitful cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-saied, H.A., Shahr El-Din, A.M., Masry, B.A. et al. A Promising Superabsorbent Nanocomposite Based on Grafting Biopolymer/Nanomagnetite for Capture of 134Cs, 85Sr and 60Co Radionuclides. J Polym Environ 28, 1749–1765 (2020). https://doi.org/10.1007/s10924-020-01720-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01720-z