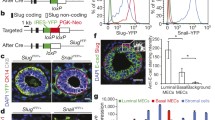
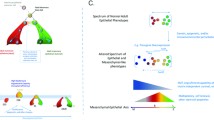
Abstract
Tissue microenvironments, also known as stem cell niches, influence not only resident cells but also cells in surrounding tissues. Physical and biochemical intercellular signals originating from resident stem cells or non-stem cells participate in the homeostasis of the tissue regulating cell proliferation, differentiation, wound healing, tissue remodeling, and tumorigenesis. In recent publications it has been demonstrated that the normal mouse mammary microenvironment can provide development and differentiation guidance to not only resident mammary cells but also cells of non-mammary origin including tumor-derived cells. When placed in reforming mammary stem cell niches the non-mammary cells proliferate and differentiate along mammary epithelial cell lineages and contribute progeny to reforming mammary gland outgrowths. The tumor-derived cells that are redirected to assume mammary epithelial phenotypes lose their cancer-forming capacity and shift their gene expression profiles from a cancer profile towards a normal mammary epithelial expression profile. This review summarizes the recent discoveries regarding the ability of the normal mouse mammary microenvironment to dictate the cell fates of non-mammary cells introduced into mammary stem cell niches.



Similar content being viewed by others
Abbreviations
- BMSC:
-
Bone marrow-derived stem cell
- CMDS:
-
Classic multidimensional scaling
- CNS:
-
Central nervous system
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Estrogen receptor
- ESC:
-
Embryonic stem cell
- HER:
-
Human epidermal growth factor receptor
- K:
-
keratin
- LTR:
-
Long terminal repeat
- MEC:
-
mammary epithelial cell
- MMP:
-
Matrix metalloproteinase
- MMTV:
-
Mouse mammary tumor virus
- NSC:
-
Neural stem cell
- NT2:
-
NTERA-2 cl
- TEB:
-
Terminal endbud
- TDLU:
-
Terminal ductal lobular unit
- TGF:
-
Transforming growth factor
- TNBC:
-
Triple negative breast cancer
- TSC:
-
Testicular-derived stem cell
- WAP:
-
Whey acidic protein promoter
References
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31.
Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803.
McBryan J, Howlin J. Pubertal mammary gland development: elucidation of in vivo morphogenesis using murine models. Methods Mol Biol. 2017;1501:77–114.
DeOme KB, Faulkin LJ Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19(5):515–20.
Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ Jr. The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968;61(1):53–60.
Daniel CW, Deome KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149(3684):634–6.
Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat. 2013;26(1):29–48.
Medina D. Stromal fibroblasts influence human mammary epithelial cell morphogenesis. Proc Natl Acad Sci U S A. 2004;101(14):4723–4.
Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11(4):R62.
Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev Biol. 2010;344(2):968–78.
Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223(4):459–68.
Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–25.
Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95(9):5076–81.
Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6(6):568–77.
Pierce DF Jr, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7(12A):2308–17.
Flanders KC, Wakefield LM. Transforming growth factor-(beta)s and mammary gland involution; functional roles and implications for cancer progression. J Mammary Gland Biol Neoplasia. 2009;14(2):131–44.
Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168(1):47–61.
Bruno RD, Smith GH. A potential mechanism for extracellular matrix induction of breast cancer cell normality. Breast Cancer Res. 2014;16(1):302.
Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106(4):619–33.
Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171(4):717–28.
Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–38.
Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11(1):R7.
Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104(10):3871–6.
Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105(39):14891–6.
Boulanger CA, Bruno RD, Mack DL, Gonzales M, Castro NP, Salomon DS, et al. Embryonic stem cells are redirected to non-tumorigenic epithelial cell fate by interaction with the mammary microenvironment. PLoS One. 2013;8(4):e62019.
Boulanger CA, Bruno RD, Rosu-Myles M, Smith GH. The mouse mammary microenvironment redirects mesoderm-derived bone marrow cells to a mammary epithelial progenitor cell fate. Stem Cells Dev. 2012;21(6):948–54.
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23(41):6980–5.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19(8):968–88.
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89(22):10578–82.
Booth BW, Boulanger CA, Anderson LH, Smith GH. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene. 2010;30(6):679–89.
Bussard KM, Smith GH. Human breast cancer cells are redirected to mammary epithelial cells upon interaction with the regenerating mammary gland microenvironment in-vivo. PLoS One. 2012;7(11):e49221.
Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–43.
Park JP, Blanding WM, Feltracco JA, Booth BW. Validation of an in vitro model of erbB2 cancer cell redirection. In Vitro Cell Dev Biol Anim. 2015;51:776–86.
Roche K, Feltus FA, Park JP, Coissieux MM, Chang C, Chan VBS, et al. Cancer cell redirection biomarker discovery using a mutual information approach. PLoS One. 2017;12(6):e0179265.
Schmucker HS, Park JP, Coissieux MM, Bentires-Alj M, Feltus FA, Booth BW. RNA expression profiling reveals differentially regulated growth factor and receptor expression in redirected Cancer cells. Stem Cells Dev. 2017;26(9):646–55.
Bruno RD, Fleming JM, George AL, Boulanger CA, Schedin P, Smith GH. Mammary extracellular matrix directs differentiation of testicular and embryonic stem cells to form functional mammary glands in vivo. Sci Rep. 2017;7:40196.
Byron A, Humphries JD, Humphries MJ. Defining the extracellular matrix using proteomics. Int J Exp Pathol. 2013;94(2):75–92.
Tong J, Mou S, Xiong L, Wang Z, Wang R, Weigand A, et al. Adipose-derived mesenchymal stem cells formed acinar-like structure when stimulated with breast epithelial cells in three-dimensional culture. PLoS One. 2018;13(10):e0204077.
Bruno RD, Reid J, Sachs PC. The revolution will be open-source: how 3D bioprinting can change 3D cell culture. Oncotarget. 2019;10(46):4724–6.
Mollica PA, Booth-Creech EN, Reid JA, Zamponi M, Sullivan SM, Palmer XL, et al. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019;95:201–13.
Reid JA, Palmer XL, Mollica PA, Northam N, Sachs PC, Bruno RD. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci Rep. 2019;9(1):7466.
Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, et al. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst. 1993;85(21):1725–32.
Rauner G, Leviav A, Mavor E, Barash I. Development of foreign mammary epithelial morphology in the Stroma of Immunodeficient mice. PLoS One. 2013;8(6):e68637.
Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1(1):206–14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frank-Kamenetskii, A., Booth, B.W. Redirecting Normal and Cancer Stem Cells to a Mammary Epithelial Cell Fate. J Mammary Gland Biol Neoplasia 24, 285–292 (2019). https://doi.org/10.1007/s10911-019-09439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-019-09439-x