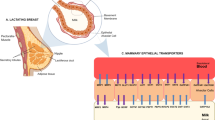
Abstract
Obesity is the most common metabolic disease whose prevalence is increasing worldwide. This condition is considered a serious public health problem due to associated comorbidities such as diabetes mellitus and hypertension. Perinatal morbidity related to obesity does not end with birth; this continues affecting the mother/infant binomial and could negatively impact on metabolism during early infant nutrition. Nutrition in early stages of growth may be essential in the development of obesity in adulthood, supporting the concept of “nutritional programming”. For this reason, breastfeeding may play an important role in this programming. Breast milk is the most recommended feeding for the newborn due to the provided benefits such as protection against obesity and diabetes. Health benefits are based on milk components such as bioactive molecules, specifically hormones involved in the regulation of food intake. Identification of these molecules has increased in recent years but its action has not been fully clarified. Hormones such as leptin, insulin, ghrelin, adiponectin, resistin, obestatin and insulin-like growth factor-1 copeptin, apelin, and nesfatin, among others, have been identified in the milk of normal-weight women and may influence the energy balance because they can activate orexigenic or anorexigenic pathways depending on energy requirements and body stores. It is important to emphasize that, although the number of biomolecules identified in milk involved in regulating food intake has increased considerably, there is a lack of studies aimed at elucidating the effect these hormones may have on metabolism and development of the newborn. Therefore, we present a state-of-the-art review regarding bioactive compounds such as hormones secreted in breast milk and their possible impact on nutritional programming in the infant, analyzing their functions in appetite regulation.

Similar content being viewed by others
Abbreviations
- BMI:
-
body mass index
- EGF:
-
epidermal growth factor
- PDGF:
-
platelet-derived growth factor
- IGF-1:
-
insulin-like growth factor-1
- GLP-1:
-
glucagon-like peptide 1
- WHO:
-
World Health Organization
- UNICEF:
-
United Nations International Children’s Emergency Fund
- ARC:
-
arcuate nucleus
- AgRP:
-
Agouti-related protein
- NYP:
-
neuropeptide Y
- POMC:
-
pro-opiomelanocortin
- CART:
-
cocaine- and amphetamine-regulated transcript
References
Harrison T. Principios de Medicina Interna.15ª Ed. New York: McGraw-Hill; 2002.
Schwartz MW. Central nervous system regulation of food intake. Obesity. 2006;14(2):1–8.
Patel MS, Srinivasan M. Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr. 2010;140(3):658–61.
Ross MG, Desai M. Developmental programming of appetite/satiety. Ann Nutr Metab. 2014;64(1):36–44.
Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009:327505.
Vickers MH. Developmental programming and transgenerational transmission of obesity. Ann Nutr Metab. 2014;64(1):26–34.
Arenz S, Rückerl R, Koletzko B, Von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes. 2004;28(10):1247–56.
Plagemann A, Harder T. Breast feeding and the risk of obesity and related metabolic diseases in the child. Metab Syndr Relat Disord. 2005;3(3):222–32.
Fields D, George B, Williams M, Whitaker K, Allison D, Teague A, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017; doi:10.1111/ijpo.12182.
Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:1–13.
Ley S, O’Connor D, Retnakaran R, Hamilton J, Sermer M, Zinman B. Impact of maternal metabolic abnormalities in pregnancy on human milk and subsequent infant metabolic development: methodology and design. BioMed Central Public Health. 2010;10(1):590.
Wen X, Triche EW, Hogan JW, et al. Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics. 2011;127:e713–21.
McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Investig. 2009;119(2):323–35.
Rolland-Cachera M, Deheeger M, Akrout M, Bellisle F. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. J Int Assoc Study Obes. 1995;19(8):573–8.
Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003;19(1):9–18.
Haschke F, Steenhout P, Grathwohl D, Haschke-Becher E. Evaluation of growth and early infant feeding: a challenge for scientists, industry and regulatory bodies. World Rev Nutr Diet. 2013;6:33–8.
Harding J. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30(1):15–23.
World Health Organization (WHO) (2015) http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/Accessed 25 January 2017.
Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151(2):702–13.
Stark R, Ashley SE, Andrews ZB. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol. 2013;366(2):215–23.
Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology. 2010;151(4):1598–610.
Gupta A, Srinivasan M, Thamadilok S, et al. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol. 2009;200:293–300.
Johnstone L, Higuchi T. Food intake and leptin during pregnancy and lactation. Prog Brain Res. 2001;133:215–27.
Wells J. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22:1–17.
Ballard J, Morrow A. Milk Composition: Nutrients and Bioactive Factors. Pediatr Clin N Am. 2013;60(1):49–74.
Picciano M. Nutrient composition of human milk. Pediatr Clin N Am. 2001;48(1):53–67.
Nommsen-Rivers L, Dolan L, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 2012;7(1):43–9.
Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Nutritional and biochemical properties of human milk: II. Lipids, micronutrients, and bioactive factors. Clin Perinatol. 1999;26(2):335–59.
Henderson J, Hartmann P, Newnham J, Simmer K. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics. 2008;121(1):92–100.
Skibiel A, Downing L, Orr T, Hood W. The evolution of the nutrient composition of mammalian milks. J Anim Ecol. 2013; doi:10.1111/1365-2656.12095.
Prentice P, Ong K, Schoemaker M, van Tol E, Vervoort J, Hughes I. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016;105(6):641–7.
Jevitt C, Hernandez I, Groër M. Lactation complicated by overweight and obesity: Supporting the mother and newborn. J Midwifery Women’s Health. 2007;149(2):185–91.
Rasmussen K, Kjolhede C. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–71.
Khodabakhshi A, Ghayour M, Rooki H, Vakili R, Hashemy S, Mirhafez S. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. Eur J Clin Nutr. 2015;69(5):614–8.
Dewey K, Nommsen-Rivers L, Heinig M, Cohen R. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3Pt 1):607–19.
Kronborg H, Vaeth M, Rasmussen K. Obesity and early cessation of breastfeeding in Denmark. Eur J Public Health. 2012;3(2):316–22.
Mäkelä J, Linderborg K, Niinikoski H, Baoru Y, Lagström H. Breast milk fatty acid composition differs between overweight and normal weight women: the STEPS Study. Eur J Nutr. 2013;52:727–35.
Castillo H, Santos I, Matijasevich A. Maternal pre-pregnancy BMI, gestational weight gain and breastfeeding. Eur J Clin Nutri. 2016;70(4):431–6.
Casabiell X, Pineiro V, Tome M, Peino R, Dieguez C, Casanueva F. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82(12):4270–3.
Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10(1):67–73.
Kon I, Shilina N, Gmoshinskaya M, Ivanushkina T. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight Ggain in breast-fed infants. Ann Nutr Metab. 2014;65(4):317–23.
Perry B, Wang Y. Appetite regulation and weight control: the role of gut hormone. Nutrit Diabetes. 2012;2(1):e26.
Newburg D, Woo J, Morrow A. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010;156(2):41–6.
Dündar N, Dündar B, Cesur G, Yilmaz N, Sütçu R, Ozgüner F. Ghrelin and adiponectin levels in colostrum, cord blood and maternal serum. Pediatr Int. 2010a;52(4):622–5.
Whitmore T, Trengove N, Graham D, Hartmann P. Analysis of insulin in human breast milk in mothers with Type 1 and Type 2 diabetes mellitus. Int J Endocrinol. 2012;1:1–9.
Fields D, Demerath E. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7(4):304–12.
Ahuja S, Boylan M, Hart S, Shriver R, Spallholz J, Pence B. Glucose and insulin levels are increased in obese and overweight mothers’ breast-milk. Food Nutr Sci. 2011;2(3):201–6.
Steculorum S, Collden G, Coupe B, Croizier S, Lockie S, Andrews Z. Neonatal ghrelin programs development of hypothalamic feeding circuits. J Clin Investig. 2015;125(2):846–58.
Whatmore AJ, Hall CM, Jones J, Westwood M, Clayton PE. Ghrelin concentrations in healthy children and adolescents. Clin Endocrinol. 2003;59(5):649–54.
Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31(12):2236–40.
Grönberg M, Tsolakis A, Magnusson L, Janson E, Saras J. Distribution of obestatin and ghrelin in human tissues immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. J Histochem Cytochem. 2008;56(9):793–801.
Granata R, Gallo D, Luque RM, Baragli A, Scarlatti F, Grande C. Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. Fed Am Soc Exp Biol J. 2012;26(8):3393–411.
Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R. Presence of obestatin in breast milk: relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition. 2008;24(7–8):689–93.
Savino F, Lupica M, Benetti S, Petrucci E, Liguori S, Cordero Di Montezemolo L. Adiponectin in breast milk: relation to serum adiponectin concentration in lactating mothers and their infants. Acta Paediatr. 2012;101(10):1058–62.
Savino F, Benetti S, Lupica MM, Petrucci E, Palumeri E. Cordero di Montezemolo L. Ghrelin and obestatin in infants, lactating mothers and breast milk. Horm Res Paediatr. 2012;78(5–6):297–303.
Savino F, Sorrenti M, Benetti S, Lupica M, Liguori S, Oggero R. Resistin and leptin in breast milk and infants in early life. Early Hum Dev. 2012;88:779–82.
Jamaluddin M, Weakley S, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165(3):622–32.
Ilcol Y, Hizli Z, Eroz E. Resistin is present in human breast milk and it correlates with maternal hormonal status and serum level of C-reactive protein. Clin Chem Lab Med. 2008;46(1):118–24.
Kelly DP. Medicine. Irisin, light my fire. Science. 2012;336(6077):42–3.
Aydin S, Kuloglu T, Aydin S. Copeptin, adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus. Peptides. 2013;47:66–70.
Repaske D, Medlej R, Gültekin E, Krishnamani M, Halaby G, Findling J. Heterogeneity in clinical manifestation of autosomal dominant of neurohypophyseal diabetes insipidus caused by a mutation encoding Ala-1->Val in the signal peptide of the arginine vasopressin/neurophysin II/copeptin precursor. J Clin Endocrinol Metab. 1997;82(1):51–6.
Morgenthaler N. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9.
Khan S, Dhillon O, O’Brien R, Struck J, Quinn P, Morgenthaler N. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester acute myocardial infarction peptide (LAMP) study. Circulation. 2007;115(16):2103–10.
Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum BBA. Biochim Biophys Acta, Mol Cell Res. 1999;1452(1):25–35.
Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A. Apelin, a Newly Identified Adipokine Up-Regulated by Insulin and Obesity. Endocrinology. 2006;146(4):1764–71.
Castan-Laurell I, Vitkova M, Daviaud, Dray C, Kovaciková M, Kovacova Z, Hejnova J, Stich V, Valet P. Effect of hypocaloric die-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. European Journal of Endocrinology. 2008;158(6):905–910.
Dong J, Guan H, Jiang Z, Chen X. Nesfatin-1 influences the excitability of glucosensing Nneurons in the dorsal vagal complex and inhibits food intake. Public Library SciOne. 2014;9(6):1–9.
Schirra J, Burkhard G. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128(2):109–15.
Schueler J, Alexander B, Hart A, Austin K, Larson E. Presence and dynamics of leptin, GLP-1, and PYY in human breast milk at early postpartum. Obesity. 2013;21(7):1451–8.
Elmlinger M, Hochhaus F, Loui A, Frommer K, Obladen M, Ranke K. Insulin-like growth factors and binding proteins in early milk from mothers of preterm and term infants. Horm Res Paediatr. 2007;68(3):124–31.
Ohkawa N, Shoji H, Kitamura T, Suganuma H, Yoshikawa N, Suzuki M, Lee T, Hisata T, Shimiz T, Hisata K, Shimizu T. IGF-leptin and active ghrelin levels in very low birth weight infants during the first 8 weeks of life. Acta Paediatrica. 2010;99:37–41.
Oddy W. Infant feeding and obesity risk in the child. Breastfeed Rev. 2012;20(2):7.
Rose’Meyer R. A review of the serotonin transporter and prenatal cortisol in the development of autism spectrum disorders. Mol Autism. 2013;4(1):37.
Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutrit Metab Care. 2013;16:320–7.
Hahn-Holbrook J, Le T, Chung A, Davis E, Glynn L. Cortisol in human milk predicts child BMI. Obesity. 2016;24(12):2471–4.
Bronsky J, Mitrova K, Karpisek M, Mazoch J, Durilova M, Fisarkova B, et al. Adiponectin, AFABP, and leptin in human breast milk during 12 months of lactation. J Pediatr Gastroenterol Nutr. 2011;52:474–7.
Ley S, Hanley A, Sermer M, Zinman B, O’Connor D. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am J Clin Nutr. 2012;95:867–74.
Larson-Meyer D, Schueler J, Kyle E, Austin K, Hart A, Alexander B. Do Lactation-Induced Changes in Ghrelin, Glucagon-Like Peptide-1, and Peptide YY Influence Appetite and Body Weight Regulation during the First Postpartum Year? J Obes. 2016;2016.
Van der Voorn B, de Waard M, van Goudoever J, Rotteveel J, Heijboer A, Finken M. Breast-milk cortisol and cortisone concentrations follow the diurnal rhythm of maternal hypothalamus-pituitary-adrenal axis activity. J Nutr. 2016;146(11):2174–9.
Hamosh M. Digestion in the newborn. Clin Perinatol. 1996;23(2):191–209.
Morán R, Naveiro R, Blanco F, Cabañeros A, Rodríguez F, Peral C. Prevalencia y duración de la lactancia materna. Influencia sobre el peso y la morbilidad. Nutr Hosp. 2009;24:213–7.
Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth. Pune maternal nutrition study. J Nutr. 2001;131(4):1217–24.
Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am J Clin Nutr. 2003;77(4):931–6.
Haschke F, Ziegler E, Grathwohl D. Fast Growth of Infants of Overweight Mothers: Can It Be Slowed Down? Ann Nutr Metab. 2014;64(1):19–24.
Çatlı G, Olgac N, Dündar B. Adipokines in breast milk: an update. J Clin Res Pediatr Endocrinol. 2014;6(4):192–201.
Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70(6):633–7.
Smith-Kirwin S, O’Connor D, Johnston J, de Lancy E, Hassink S, Funanage V. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83(5):1810.
Bonnet M, Gourdou I, Leroux C, Chilliard Y, Djiane J. Leptin expression in the ovine mammary gland: putative sequential involvement of adipose, epithelial, and myoepithelial cells during pregnancy and lactation. J Anim Sci. 2002;80(3):723–8.
Miralles O, Sánchez J, Palou A, Picó C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity. 2006;14(8):1371–7.
Casabiell X, Pineiro V, Tome M, Peino R, Dieguez C, Casanueva F. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82(12):4270–3.
Savino F, Fissore M, Grassino E, Nanni G, Oggero R, Silvestro L. Ghrelin, leptin and IGF-I levels in breast-fed and formula-fed infants in the first years of life. Acta Pediátr. 2005;94(5):531–7.
López M, Tovar S, Vázquez M, Williams L, Diéguez C. Peripheral tissue-brain interactions in the regulation of food intake. Proc Nutr Soc. 2007;66(1):131–55.
Andreas N, Hyde M, Gale C, Parkinson J, Jeffries S, Holmes E. Effect of Maternal Body Mass Index on Hormones in Breast Milk: A Systematic Review. Public Library Sci One. 2014;9(12):1–25.
Woo J, Guerrero M, Guo F, Martin L, Davidson BS, Ortega H. Human milk adiponectin affects infant weight trajectory during the second year of life. J Pediatr Gastroenterol Nutr. 2012;54(4):532–9.
Ozarda Y, Gunes Y, Tuncer G. The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clin Chem Lab Med. 2012;50(5):911–7.
Jovanovic-Peterson L, Fuhrmann K, Hedden K, Walker L, Peterson C. Maternal milk and plasma glucose and insulin levels: studies in normal and diabetic subjects. J Am Coll Nutr. 1989;8(2):125–31.
Kirk S, Samuelsson A, Argenton M. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. Public Library SciOne. 2009;4(6):e5870.
Jamaluddin M, Weakley S, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165(3):622–32.
Mora I, Espinoza A, López N, Acevo P, Romero FM, Montero P. Indicadores de riesgo cardiovascular, patrones de lactancia y estilo de vida de la madre durante el proceso de crecimiento y desarrollo fetal e infantil. Nutr Clín Diet Hosp. 2015;35(2):91–100.
Dobša L, Cullen K. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172–90.
Saleem U, Khaleghi M, Morgenthaler N, Bergmann A, Struck J, Mosley T. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. The J Clin Endocrinol Metab. 2009;94(7):2558–64.
Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler N, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(7):1065–72.
Castan-Laurell I, Vitkova M. Daviaud, Dray C, Kovaciková M, Kovacova Z, Hejnova J, Stich V, Valet P. Effect of hypocaloric die-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158(6):905–10.
Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum BBA. Biochim Biophys Acta Mol Cell Res. 1999;1452(1):25–35.
Stanley T, Feldpausch M, Murphy C, Grinspoon S, Makimura H. Discordance of IGF-1 and GH Stimulation Testing for Altered GH Secretion in Obesity. Growth Hormon IGF Res. 2014;24(1):10–5.
Ohkawa N, Shoji H, Kitamura T, Suganuma H, Yoshikawa N, Suzuki M. IGF-leptin and active ghrelin levels in very low birth weight infants during the first 8 weeks of life. Acta Paediatr. 2009;99:37–41.
Hinde K, Skibiel A, Foster A, Del Rosso L, Mendoza S, Capitanio J. Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol. 2014;26:269–81.
Jwa S, Fujiwara T, Kondo N. Latent protective effects of breastfeeding on late childhood overweight and obesity: a nationwide prospective study. Obesity. 2014;22:1527–37.
Carter M, Dudley D, Nathanielsz P. Fetal cortisol is evaluated in maternal obesity (MO). Reprod Sci. 2011;18:139A.
Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstetr Gynecol. 2009;2(4):222–31.
Bernstein RM, Hinde, K. Bioactive factors in milk across lactation: Maternal effects and influence on infant growth in rhesus macaques (Macaca mulatta). Am J Primatol. 2016; 78(8).
Power ML, Schulkin J, Drought H, Milligan LA, Murtough KL, Bernstein RM. Patterns of milk macronutrients and bioactive molecules across lactation in a western lowland gorill a (Gorilla gorilla) and a Sumatran orangutan (Pongo abelii). Am J Primatol. 2016;9999:1–11.
Quinn EA, Geoff C. Ecological pressures and milk metabolic hormones of ethnic Tibetans living at different altitudes. Ann Hum Biol. 2017;44(1):34–45.
Fields DA, Camille RS, Gregory P. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity. 2016: 1213–1221.
Neu J. Gastrointestinal maturation and implications for infant feeding. Early Hum Dev. 2007;83(12):167–775.
Acknowledgements
This research was supported by the Coordinación de Investigación Médica en Salud, IMSS, México (Grant #FIS/IMSS/PROT/PRIO/15/045.
The authors acknowledge Sharon Morey, Scientific Communications, for providing editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badillo-Suárez, P.A., Rodríguez-Cruz, M. & Nieves-Morales, X. Impact of Metabolic Hormones Secreted in Human Breast Milk on Nutritional Programming in Childhood Obesity. J Mammary Gland Biol Neoplasia 22, 171–191 (2017). https://doi.org/10.1007/s10911-017-9382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-017-9382-y