Abstract
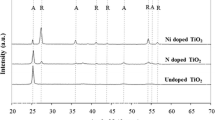
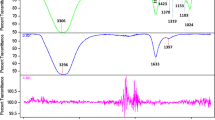
In this paper we report the antibacterial activity in the absence of UV–Vis irradiation of TiO2 nanoparticles, in amorphous, anatase and rutile phases, obtained by the sol–gel process, on Escherichia coli strains. The synthesized TiO2 powders were characterized using X-ray diffraction (XRD), IR spectroscopy and UV–Vis absorption, as well as scanning and transmission electron microscopies. The XRD results showed that the solids were amorphous up to a temperature of 350 °C and that when subjected to heat treatments of higher temperatures, anatase crystalline phases were obtained, at 450 °C, and rutile type at temperatures higher than 770 °C, with a sub-micron particle size (< 1 μm) and varying morphology. The inactivating effect on bacteria of synthesized TiO2 was analyzed by recording the effect of its presence on bacterial strains of E. coli. To this end, the synthesized TiO2 in its amorphous (am-TiO2), anatase (a-TiO2) or rutile (r-TiO2) phases, at different concentrations, was incorporated into the E. coli cultures, placing aluminum foil over the strains to simulate darkness. Although all the phases of the TiO2 synthesized present reasonable antibacterial activity, the highest efficiency is seen in the cultures treated with r-TiO2.






Similar content being viewed by others
References
J. F. Banfield and D. R. Veblen (1992). Am. Miner. 77, 545–557.
W. H. Bauer (1961). Acta Crystallogr. A 14, 214–216. https://doi.org/10.1107/S0365110x61000747.
G. V. Samsonov The Oxide Handbook (Plenum Press, New York, 1982).
X. Bokhimi, A. Morales, M. Aguilar, J. A. Toledo-Antonio, and F. Pedraza (2001). Int. J. Hydrogen Energy 26, 1279–1287. https://doi.org/10.1016/S0360-3199(01)00063-5.
F. A. Grant (1959). Rev. Mod. Phys. 31, 646–674. https://doi.org/10.1103/RevModPhys.31.646.
L. Brohan, A. Verbaere, M. Tournoux, and G. Demazeau (1982). Mater. Res. Bull. 17, 355–361. https://doi.org/10.1016/0025-5408(82)90085-X.
A. G. Dylla, G. Henkelman, and K. J. Stevenson (2013). Acc. Chem. Res. 46, 1104–1112. https://doi.org/10.1021/ar300176y.
L. S. Dubrovinsky, N. A. Dubrovinskaia, V. Swamy, J. Muscat, N. M. Harrison, R. Ahuja, B. Holm, and B. Johansson (2001). Nature 410, 653–654. https://doi.org/10.1038/35070650.
J. Akimoto, Y. Gotoh, Y. Oosawa, N. Nonose, T. Kumagai, K. Aoki, and H. Takei (1994). J. Solid State Chem. 113, 27–36. https://doi.org/10.1006/jssc.1994.1337.
M. Mattesini, J. S. D. Almeida, L. Dubrovinsky, N. Dubrovinskaia, B. Johansson, and R. Ahuja (2004). Phys. Rev. B 70, 212110. https://doi.org/10.1103/physRevB.70.212101.
M. Latroche, L. Brohan, R. Marchand, and M. Tournoux (1989). J. Solid State Chem. 81, 78–82. https://doi.org/10.1016/0022-4596(89)90204-1.
J. K. Dewhurst and J. E. Lowther (1996). Phys. Rev. B 54, R3673. https://doi.org/10.1103/PhysRevB.54.R3673.
M. Gopel, W. J. Moberly Chan, and L. C. De Jonghe (1997). J. Mater. Sci. 32, 6001–6008. https://doi.org/10.1023/a:1018671212890.
S. G. Kumar and K. S. Rao (2014). Nanoscale 6, 11574–11632. https://doi.org/10.1039/c4nr01657b.
U. Bach, Y. Tachinaba, J. E. Moser, S. A. Haque, J. R. Durrant, M. Graetzel, and D. R. Klug (1999). J. Am. Chem. Soc. 121, 7445–7446. https://doi.org/10.1021/ja9915403.
N. G. Park, J. Van de Lagemaat, and A. J. Frank (2000). J. Phys. Chem. B 104, 8989–8994. https://doi.org/10.1021/jp9943651.
M. Kaneko and I. Okura Photocatalysis: Science and Technology (Kodansha-Spring Verlag, New York, 2002).
M. Anpo and P. V. Kamat Environmentally Benign Photocatalysts (Springer, New York, 2010).
G. Nogami, R. Shiratsuchi, and S. Ohkubo (1991). J. Electrochem. Soc. 138, 751–758. https://doi.org/10.1149/1.2085670.
E. Topoglidis, A. E. Cass, G. Gilardi, S. Sadeghi, N. Beaumont, and J. R. Durrant (1998). Anal. Chem. 70, 5111–5113. https://doi.org/10.1021/ac980764l.
J. Geserick, T. Froeschl, N. Huesing, G. Kucerova, M. Makosch, T. Diemant, S. Ecckle, and R. J. Behm (2011). Dalton Trans. 40, 3269–3286. https://doi.org/10.1039/C0DT00911C.
Z. Zhang, A. Kladi, and X. E. Verykios (1994). J. Phys. Chem. 98, 6804–6811. https://doi.org/10.1021/j100078a024.
M. M. Shubert, V. Plzak, J. Garche, and R. J. Behm (2001). Catal. Lett. 76, 143–150. https://doi.org/10.1023/A:1012365710979.
W. P. Hsu, R. Yu, and E. Matijevic (1993). J. Colloid Interface Sci. 156, 56–65. https://doi.org/10.1006/jcis.1993.1080.
P. Kubiak, T. Froeschl, N. Huesing, U. Hoermann, U. Kaiser, R. Schiller, C. K. C. K. Weiss, K. Landfester, and M. Wohlfahrt-Mhrens (2011). Small 7, 1690–1696. https://doi.org/10.1002/smll.201001943.
L. Kavan (2012). Chem. Rev. 12, 131–142. https://doi.org/10.1002/tcr.201100012.
A. Mills, H. R. Davis, and D. Worsley (1993). Chem. Soc. Rev. 22, 417–425. https://doi.org/10.1039/CS9932200417.
P. Pichat Photocatalysis and Water Purification (Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim, 2013). https://doi.org/10.1002/9783527645404.
P. C. Maness, S. Smolinski, D. M. Blake, Z. Huang, E. J. Wolfrum, and W. A. Jacoby (1999). Appl. Environ. Microbiol. 65, 4094–4098.
N. Cioffi and M. Rai Nano-antimicrobials: Progress and Prospects in Section I Synthesis and Characterization of Novel Nanomicrobials (Springer, Berlin, 2012).
Y. Paz, Z. Luo, L. Rabenberg, and A. Heller (1995). J. Mater. Res. 10, 2842–2848. https://doi.org/10.1557/JMR.1995.2842.
G. Eranna Metal Oxide Nanostructures as Gas Sensing Devices (Taylor & Francis, Boca Raton, 2012).
X. Chen and S. S. Mao (2007). Chem. Rev. 107, 2891–2959. https://doi.org/10.1021/cr0500535.
T. Fröschl, U. Hörmann, P. Kubiak, G. Kucerová, M. Pfanzelt, C. K. Eiss, R. J. Behm, N. Hüsing, U. Kaiser, K. Landfester, and M. Wohlfahrt-Mehrens (2012). Chem. Soc. Rev. 41, 5313–5360. https://doi.org/10.1039/C2CS35013K.
Y. Bai, I. Mora-Seró, F. D. Angelis, J. Bisquert, and P. Wang (2014). Chem. Rev. 114, 1095–10130. https://doi.org/10.1021/cr400606n.
Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han, and C. Li (2014). Chem. Rev. 114, 9987–10043. https://doi.org/10.1021/cr500008u.
U. Diebold (2003). Surf. Sci. Rep. 48, 53–229. https://doi.org/10.1016/S0167-5729(02)00100-0.
V. V. Hong, H. Zung, and N. H. B. Trong (2007). Eur. Phys. J. D 44, 515–524. https://doi.org/10.1140/epjd/e2007-00186-5.
H. Zhang, B. Chen, and J. F. Banfield (2008). Phys. Rev. B 78, 214106. https://doi.org/10.1103/PhysRevB.78.214106.
V. V. Hong in K. S. Sattler (ed.), Handbook of Nanophysics: Nanoparticles and Quantum Dots (CRC Press, New York, 2010), pp. 1-1–1-10.
V. V. Hong (2011). Chem. Phys. Res. J. 4, 43–62.
L. Romano, V. Privitera, and C. Jagadish Defects in Semiconductors, Semiconductors and Semimatels, vol. 91 (Academic Press, San Diego, 2015).
U. I. Gaya Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids (Springer, Dordrecht, 2014).
M. K. Nowotny, L. R. Sheppard, and J. Nowotny (2008). J. Phys. Chem. C 112, 5275–5300. https://doi.org/10.1021/jp077275m.
X. Pan, M. Q. Yang, X. Fu, N. Zhang, and Y. J. Xu (2013). Nanoscale 5, 3601–3614. https://doi.org/10.1039/c3nr00476g.
Z. Wu, S. Cao, C. Zhang, and L. Piao (2017). Nanotechnology 28, 275706. https://doi.org/10.1088/1361-6528/aa7373.
V. Gurylev, M. Mishra, Y. C. Su, and T. P. Perng (2016). Chem. Commun. 52, 7604–7607. https://doi.org/10.1039/C5CC10610A.
M. Kong, Y. Z. Li, X. Chen, T. T. Tian, P. F. Fang, F. Zheng, and X. J. Zhao (2011). J. Am. Chem. Soc. 133, 16414–16417. https://doi.org/10.1021/ja207826q.
A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, and H. Pettersson (2010). Chem. Rev. 110, 6595–6663. https://doi.org/10.1021/cr900356p.
S. C. Roy, O. K. Varghese, M. Paulose, and C. A. Grimes (2010). ACS Nano 4, 1259–1273. https://doi.org/10.1021/nn9015423.
C. C. Mercado, F. J. Knorr, J. L. McHale, S. M. Usmani, A. S. Chimura, and L. V. Saraf (2012). J. Phys. Chem. C 116, 10796–10804. https://doi.org/10.1021/jp301680d.
J. Wu, H. Lu, X. Zhang, F. Raziq, Y. Qu, and L. Jing (2016). Chem. Commun. 52, 5027–5029. https://doi.org/10.1039/C6CC00772D.
Z. Zhao, X. Zhang, G. Zhang, Z. Liu, D. Qu, X. Miao, P. Feng, and Z. Sun (2015). Nano Res. 8, 4061–4071. https://doi.org/10.1007/s12274-015-0917-5.
V. Srivastava, D. Gusain, and Y. Chandra Sharma (2015). Ind. Eng. Chem. Res. 54, 6209–6233. https://doi.org/10.1021/acs.iecr.5b01610.
N. Durán, S. S. Guterres, and O. L. Alves Nanotoxicology: Materials, Methodologies and Assessments (Springer, New York, 2014). https://doi.org/10.1007/978-1-4614-8993-1.
A. Albanese, P. S. Tang, and W. C. W. Chan (2012). Annu. Rev. Biomed. Eng. 14, 1–16. https://doi.org/10.1146/annurev-bioeng-071811-150124.
T. Matsunaga, R. Tomoda, T. Nakajima, and H. Wake (1985). FEMS Microbiol. Lett. 29, 211–214. https://doi.org/10.1111/j.1574-6968.1985.tb00864.x.
H. M. Yadav, J. S. Kim, and S. H. Pawar (2016). Korean J. Chem. Eng. 33, 1989–1998. https://doi.org/10.1007/s11814-016-0118-2.
O. Akhavan and E. Ghaderi (2010). Surf. Coat. Technol. 204, 3676–3683. https://doi.org/10.1016/j.surfcoat.2010.04.048.
F. Grande and P. Tucci (2016). Mini. Rev. Med. Chem. 16, 762–769. https://doi.org/10.2174/1389557516666160321114341.
S. M. Dizaj, F. Lotfipour, M. Barzegar-Jajali, M. H. Zarrintan, and K. Adibkla (2014). Mater. Sci. Eng. C 44, 278–284. https://doi.org/10.1016/j.msec.2014.08.031.
A. S. Roy, A. Parveen, A. R. Koppalkar, and M. Prasad (2013). J. Biomater. Nanobiotechnol. 1, 37–41. https://doi.org/10.4236/jbnb.2010.11005.
I. Fenoglio, G. Greco, S. Livraghi, and B. Fubini (2009). Chem. Eur. J. 15, 4614–4621. https://doi.org/10.1002/chem.200802542.
M. Li, M. E. Noriega-Trevino, N. Nino-Matínez, C. Marambio-Jones, J. Wang, R. Damoiseaux, F. Ruiz, and E. M. V. Hock (2011). Environ. Sci. Technol. 45, 8989–8995. https://doi.org/10.1021/es201675m.
E. Albert, P. A. Albouy, A. Ayral, P. Basa, G. Csik, N. Nagy, S. Rouldés, V. Rouessac, G. Sáfrán, A. Suhajda, Z. Zolnai, and Z. Hórvölgyi (2015). RSC Adv. 5, 59070. https://doi.org/10.1039/c5ra05990a.
I. Daou, N. Moukrad, O. Zegaoui, and F. R. Filai (2017). Water Sci. Technol. 77, 1238–1249. https://doi.org/10.2166/wst.2017.647.
K. Hirota, M. Sugimoto, M. Kato, K. Tsukagoshi, T. Tanigawa, and H. Sugimoto (2010). Ceram. Int. 36, 497–506. https://doi.org/10.1016/j.ceramint.2009.09.026.
V. L. Prasanna and R. Vijayaraghavan (2015). Langmuir 31, 9155–9162. https://doi.org/10.1021/acs.langmuir.5b002266.
A. Joe, S. H. Park, K. D. Shim, D. J. Kim, K. H. Jhee, H. W. Lee, C. H. Heo, H. M. Kim, and E.-S. Jang (2017). J. Ind. Eng. Chem. 45, 430–439. https://doi.org/10.1016/j.jiec.2016.10.013.
J. R. Gurr, A. S. S. Wang, C. H. Chen, and K. Y. Jan (2005). Toxicology 213, 66–73. https://doi.org/10.1016/j.tox.2005.
N. K. Gali, Z. Ning, W. Daoud, and P. Brimblecombe (2016). J. Appl. Toxicol. 36, 1355–1363. https://doi.org/10.1002/jat.3341.
K. Tanaka, M. F. V. Capule, and T. Hinasaga (1991). Chem. Phys. Lett. 187, 73–76. https://doi.org/10.1016/0009-2614(91)90486-S.
S. L. Suib, New and Future Developments in Catalysis: Solar Photocatalysis, chap. 10 (Elsevier, Amsterdam, 2013).
V. H. Grassian, Nanoscience and Nanotechnology: Environmental and Health Impacts, chap. 13 (Wiley, Hoboken, 2008).
A. Rincón and C. Pulgarin (2004). Appl. Catal. B Environ. 49, 99–112. https://doi.org/10.1016/j.apcatb.2003.11.013.
M. Bekbolet (1997). Water Sci. Technol. 35, 95–100. https://doi.org/10.1016/S0273-1223(97)00241-2.
M. Cho, H. Chung, W. Choi, and J. Yoon (2005). Appl. Environ. Microbiol. 71, 270–275. https://doi.org/10.1128/AEM.71.1.270-275.2005.
M. A. Vargas and J. E. Rodríguez-Páez (2017). J. Non Cryst. Solids 459, 192–205. https://doi.org/10.1016/j.jnoncrysol.2017.01.018.
K. Nakamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds (Wiley-Interscience, Hoboken, 2009).
L. Marchese, E. Gianotti, V. Dellarocca, T. Maschmeyer, F. Rey, S. Coluccia, and J. M. Thomas (1999). Phys. Chem. Chem. Phys. 1, 585–592. https://doi.org/10.1039/A808225A.
K. Sunada, T. Watanabe, and K. Hashimoto (2003). J. Photochem. Photobiol. A 156, 227–233. https://doi.org/10.1016/S1010-6030(02)00434-3.
Z. Huang, P. C. Maness, D. M. Blake, E. J. Wolfrum, S. Smolinski, and W. Jacoby (2000). J. Photochem. Photobiol. A 130, 163–170. https://doi.org/10.1016/S1010-6030(99)00205-1.
M. J. Llansola-Portoles, J. J. Bergkamp, D. Finkelstein-Shapino, B. D. Sherman, G. Kadis, N. M. Dimitrijevic, D. Gust, T. A. Moore, and A. L. Moore (2014). J. Phys. Chem. A 118, 10631–10638. https://doi.org/10.1021/jp506284q.
D. A. Panayotov and J. R. Morris (2009). J. Phys. Chem. C 113, 15684–15691. https://doi.org/10.1021/jp9036233.
L. B. Xiong, J. L. Li, B. Yang, and Y. Yu (2012). J. Nanomater.. https://doi.org/10.1155/2012/831524.
A. C. Papageorgiou, N. S. Beglitis, C. L. Pang, G. Teobaldi, G. Caballh, Q. Chen, A. J. Fisher, W. A. Hofer, and G. Thornton (2010). PNAS 107, 2391–2396. https://doi.org/10.1073/pnas.0911349107.
A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova, and M. Thompson (2009). Nat. Mater. 8, 543–557. https://doi.org/10.1038/nmat2442.
Q. X. Mu, G. B. Jiang, L. X. Chen, H. Y. Zhou, D. Fourches, A. Tropsha, and B. Yan (2014). Chem. Rev. 114, 7740–7781. https://doi.org/10.1021/cr400295a.
V. M. Longo, F. C. Picon, C. Zamperini, A. R. Albuquerque, J. R. Sambrano, C. E. Vergani, A. L. Machado, J. Andrés, A. C. Hernandes, J. A. Varela, and E. Longo (2013). Chem. Phys. Lett. 577, 114–120. https://doi.org/10.1016/j.cplett.2013.05.056.
T. Bak, J. Nowotny, N. J. Sucher, and E. Wachsman (2011). J. Phys. Chem. C 115, 15711–15738. https://doi.org/10.1021/jp2027862.
Acknowledgements
We are grateful to the University of Cauca for making their laboratory facilities available for carrying out this work and to VRI-Unicauca for all logistical support. We are especially grateful to Colin McLachlan for suggestions relating to the English text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vargas, M.A., Rodríguez-Páez, J.E. Facile Synthesis of TiO2 Nanoparticles of Different Crystalline Phases and Evaluation of Their Antibacterial Effect Under Dark Conditions Against E. coli. J Clust Sci 30, 379–391 (2019). https://doi.org/10.1007/s10876-019-01500-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01500-3