Abstract
Purpose
The first step in diagnosing hemophagocytic lymphohistiocytosis (HLH) is to suspect its presence and then order the appropriate diagnostic tests. The development of screening procedures for HLH could facilitate early diagnosis. In this study, we evaluated the utility of fever, splenomegaly, and cytopenias as screening criteria for identifying pediatric HLH at an early stage, built a screening model using commonly measured laboratory parameters, and developed a step-wise screening procedure for pediatric HLH.
Methods
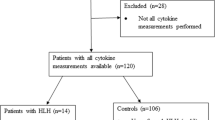
The medical records of 83,965 pediatric inpatients, including 160 patients with HLH, were collected retrospectively. The utility of fever, splenomegaly, hemoglobin level, and platelet and neutrophil counts at hospital admission as screening criteria for HLH was evaluated. For HLH patients who might be missed by screening based on the presence of fever, splenomegaly, and cytopenias, a screening model using common laboratory parameters was developed. Following that, a three-step screening procedure was then developed.
Results
The criteria of cytopenias affecting two or more lineages plus fever or splenomegaly had a sensitivity of 51.9% and a specificity of 98.4% for identifying HLH in pediatric inpatients. Our screening score model comprises six parameters: splenomegaly, platelet count, neutrophil count, albumin level, total bile acid level, and lactate dehydrogenase level. The use of the validation set had a sensitivity of 87.0% and a specificity of 90.6%. A three-step screening procedure has been developed: Step 1: Is fever or splenomegaly present? (Yes: risk for HLH should be considered, go to Step 2; No: less likely HLH); Step 2: Are cytopenias affecting at least two lineages? (Yes: consider HLH; No: go to Step 3); Step 3: Calculate the screening score. Is the sum of the score greater than 37? (Yes: consider HLH; No: less likely HLH). The overall sensitivity and specificity of the three-step screening procedure were 91.9% and 94.4%, respectively.
Conclusion
A significant proportion of pediatric HLH patients present at the hospital without having all three symptoms: fever, splenomegaly, and cytopenias. Our three-step screening procedure, utilizing commonly available clinical and laboratory parameters, can effectively identify pediatric patients who may be at high risk for HLH.



Similar content being viewed by others
Data Availability
The dataset used and analyzed in the current study is available from the corresponding author upon reasonable request.
References
Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–52. https://doi.org/10.1182/blood-2011-03-278127.
Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515. https://doi.org/10.1016/j.berh.2020.101515.
Si SJ, Tasian SK, Bassiri H, Fisher BT, Atalla J, Patel R, et al. Diagnostic challenges in pediatric hemophagocytic lymphohistiocytosis. J Clin Immunol. 2021;41(6):1213–8. https://doi.org/10.1007/s10875-021-01025-3.
Athale J. Challenges in identifying hemophagocytic lymphohistiocytosis in the ICU. Crit Care Med. 2020;48(4):599–600. https://doi.org/10.1097/ccm.0000000000004257.
Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31. https://doi.org/10.1002/pbc.21039.
Li X, Yan H, Xiao Z, Zhang X, Huang J, Xiang S-T, et al. Diagnostic time lag of pediatric haemophagocytic lymphohistiocytosis and patient characteristics: a retrospective cohort study. Front Pediatr. 2021:9. https://doi.org/10.3389/fped.2021.692849.
Marsh RA. Epstein–Barr virus and hemophagocytic lymphohistiocytosis. Front Immunol. 2018;8:1902. https://doi.org/10.3389/fimmu.2017.01902.
Risma KA, Marsh RA. Hemophagocytic lymphohistiocytosis: clinical presentations and diagnosis. J Allergy Clin Immunol Pract. 2019;7(3):824–32. https://doi.org/10.1016/j.jaip.2018.11.050.
Bin Q, Gao JH, Luo JM. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. 2016;95(9):1411–8. https://doi.org/10.1007/s00277-016-2727-6.
Luo ZB, Chen YY, Xu XJ, Zhao N, Tang YM. Prognostic factors of early death in children with hemophagocytic lymphohistiocytosis. Cytokine. 2017;97:80–5. https://doi.org/10.1016/j.cyto.2017.03.013.
Li X, Yan H, Zhang X, Huang J, Xiang S-T, Yao Z, et al. Clinical profiles and risk factors of 7-day and 30-day mortality among 160 pediatric patients with hemophagocytic lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):229. https://doi.org/10.1186/s13023-020-01515-4.
Hongmei Yang AM, Carolinas Healthcare System. A macro of building predictive model in PROC LOGISTIC with AIC-optimal variable selection embedded in cross-validation. SESUG Paper AD-36-2017. 2017. https://www.lexjansen.com/sesug/2017/APP-36.pdf.
Li X, Yan H, Xiao Z, Luo T, Xie L, Yang Y, et al. Development of a screening score for hemophagocytic lymphohistiocytosis among pediatric patients with acute infection of Epstein-Barr virus. Front Immunol. 2022:13. https://doi.org/10.3389/fimmu.2022.981251.
Smits BM, van Montfrans J, Merrill SA, van de Corput L, van Gijn M, de Vries A, et al. A Minimal parameter set facilitating early decision-making in the diagnosis of hemophagocytic lymphohistiocytosis. J Clin Immunol. 2021;41(6):1219–28. https://doi.org/10.1007/s10875-021-01005-7.
Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheum. 2014;66(9):2613–20. https://doi.org/10.1002/art.38690.
Knaak C, Nyvlt P, Schuster FS, Spies C, Heeren P, Schenk T, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. https://doi.org/10.1186/s13054-020-02941-3.
Debaugnies F, Mahadeb B, Ferster A, Meuleman N, Rozen L, Demulder A, et al. Performances of the H-score for diagnosis of hemophagocytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol. 2016;145(6):862–70. https://doi.org/10.1093/ajcp/aqw076.
Lachmann G, Spies C, Schenk T, Brunkhorst FM, Balzer F, La Rosee P. Hemophagocytic lymphohistiocytosis: potentially underdiagnosed in intensive care units. Shock. 2018;50(2):149–55. https://doi.org/10.1097/SHK.0000000000001048.
Buyse S, Teixeira L, Galicier L, Mariotte E, Lemiale V, Seguin A, et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–702. https://doi.org/10.1007/s00134-010-1936-z.
Debaugnies F, Mahadeb B, Nagant C, Meuleman N, De Bels D, Wolff F, et al. Biomarkers for early diagnosis of hemophagocytic lymphohistiocytosis in critically ill patients. J Clin Immunol. 2021;41(3):658–65. https://doi.org/10.1007/s10875-020-00950-z.
Lin H, Scull BP, Goldberg BR, Abhyankar HA, Eckstein OE, Zinn DJ, et al. IFN-gamma signature in the plasma proteome distinguishes pediatric hemophagocytic lymphohistiocytosis from sepsis and SIRS. Blood Adv. 2021;5(17):3457–67. https://doi.org/10.1182/bloodadvances.2021004287.
Cui Y, Xiong X, Ren Y, Wang F, Wang C, Zhang Y. CD163 as a valuable diagnostic and prognostic biomarker of sepsis-associated hemophagocytic lymphohistiocytosis in critically ill children. Pediatr Blood Cancer. 2019;66(10):e27909. https://doi.org/10.1002/pbc.27909.
Koh KN, Im HJ, Chung NG, Cho B, Kang HJ, Shin HY, et al. Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: report of a nationwide survey from Korea Histiocytosis Working Party. Eur J Haematol. 2015;94(1):51–9. https://doi.org/10.1111/ejh.12399.
Batu ED, Erden A, Seyhoğlu E, Kilic L, Büyükasık Y, Karadag O, et al. Assessment of the HScore for reactive haemophagocytic syndrome in patients with rheumatic diseases. Scand J Rheumatol. 2017;46(1):44–8. https://doi.org/10.3109/03009742.2016.1167951.
Croden J, Grossman J, Sun H. External validation of the HLH-2004 diagnostic criteria and H-score for diagnosis of hemophagocytic lymphohistiocytosis in adults. Blood. 2020;136:44–5. https://doi.org/10.1182/blood-2020-138589.
Bilston L, Croden J, Taparia M, Karkhaneh M, Grossman J, Sun HL. Validation of the HScore and the HLH-2004 diagnostic criteria for the diagnosis of hemophagocytic lymphohistiocytosis in a multicenter cohort. Eur J Haematol. 2022;109(2):129–37. https://doi.org/10.1111/ejh.13779.
Acknowledgements
The authors would like to thank Dr. Youcai Deng for his valuable comments and suggestions. The authors would like to thank Dan Yang and Yu Tian for their assistance in data management.
Funding
This study was supported by the National Natural Science Foundation of China (Young Scientists Fund, No. 82102285, grant to XLi), the Hunan Provincial Natural Science Foundation of China (No. 2021JJ40270, grant to XLi), the Hunan Provincial Science and Technology Department Project (2020SK1014-3 and 2020SK2114, grant to XLu), the Hunan Provincial Key Laboratory of Emergency Medicine for Children (No. 2018TP1028, grant to ZX), and the Scientific Research Project of Hunan Provincial Health Commission (No. 202112050360, grant to XLi). The study sponsors have no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
HY and XLi contributed to the study’s conception, analyzed and interpreted the data, and wrote the manuscript. ZX, TL, LX, ZT, MT, LG, JH, XZ, and MZ contributed to the study design, interpreted the data, and revised the manuscript. ZY, PZ, and DZ performed chart reviews, interpreted the data, and revised the manuscript. XLu and ZX designed the study, interpreted the data, and revised the manuscript. All authors read and approved the final manuscript. The corresponding author (XLu) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Statement
The study protocol was reviewed and approved by the Medical Ethics Committee of the Hunan Children’s Hospital (HCHLL-2019-40 and HCHLL-2022-50).
Consent to Participate
Informed consent was waived by the Medical Ethics Committee of the Hunan Children’s Hospital because of the retrospective design. The authors had no access to information that could identify individual participants during and after data collection.
Consent to Publish
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
Supplementary Table S1. Distribution of investigated laboratory parameters among pediatric patients in the training set according to HLH status. Supplementary Table S2. Parameters of the multivariate logistic regression model. Supplementary Table S3. Performance of the three-step HLH screening procedure using standard and alternative scoring parameters (n=78050). (DOCX 22 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Yan, H., Xiao, Z. et al. A Three-Step Screening Procedure for Early Identification of Children at High Risk of Hemophagocytic Lymphohistiocytosis. J Clin Immunol 43, 989–998 (2023). https://doi.org/10.1007/s10875-023-01458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-023-01458-y