Abstract
Purpose
Hypomorphic mutations in RAG1 and RAG2 are associated with significant clinical heterogeneity and symptoms of immunodeficiency or autoimmunity may be late in appearance. As a result, immunosuppressive medications may be introduced that can have life-threatening consequences. We describe a previously healthy 13-month-old girl presenting with rash and autoimmune hemolytic anemia, while highlighting the importance of vigilance and consideration of an underlying severe immunodeficiency disease prior to instituting immunosuppressive therapy.
Methods
Given clinical deterioration of the patient and a temporal association with recently administered vaccinations, virus genotyping was carried out via 4 real-time Forster Resonance Energy Transfer PCR protocols targeting vaccine-associated single nucleotide polymorphisms. Genomic DNA was extracted from whole blood and analyzed via the next-generation sequencing method of sequencing-by-synthesis. Immune function studies included immunophenotyping of peripheral blood lymphocytes, mitogen-induced proliferation and TLR ligand-induced production of TNFα. Analysis of recombination activity of wild-type and mutant RAG2 constructs was performed.
Results
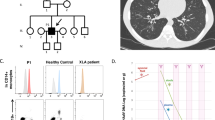
Virus genotyping revealed vaccine-strain VZV, mumps, and rubella. Next-generation sequencing identified heterozygosity for RAG2 R73H and P180H mutations. Profound lymphopenia was associated with intense corticosteroid therapy, with some recovery after steroid reduction. Residual, albeit low, RAG2 protein activity was demonstrated.
Conclusions
Because of the association of RAG deficiency with late-onset presentation and autoimmunity, live virus vaccination and immunosuppressive therapies are often initiated and can result in negative consequences. Here, hypomorphic RAG2 mutations were linked to disseminated vaccine-strain virus infections following institution of corticosteroid therapy for autoimmune hemolytic anemia.

Similar content being viewed by others
Abbreviations
- ACIP:
-
Advisory Committee on Immunization Practices
- CMV:
-
Cytomegalovirus
- Con A:
-
Concanavalin A
- CVID:
-
Common variable immune deficiency
- D:
-
Diversity
- hCD4:
-
Human CD4
- HSCT:
-
Hematopoietic stem cell transplantation
- IVIG:
-
Intravenous immunoglobulin
- J:
-
Joining
- PHA:
-
Phytohemagglutinin
- RAG:
-
Recombination-activating gene
- SCID:
-
Severe combined immune deficiency
- SD:
-
Standard deviation
- TLR:
-
Toll-like receptor
- TREC:
-
T cell receptor excision circle
- V:
-
Variable
- VZV:
-
Varicella zoster virus
References
Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232.
Kim MS, Lapkouski M, Yang W, Gellert M. Crystal structure of the V(D)J recombinase RAG1-RAG2. Nature. 2015;518:507–11.
Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–9.
Avila EM, Uzel G, Hsu A, Milner JD, Holland SM. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics. 2010;126:1248–52.
Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–6.
de Villartay JP, Lim A, Al-Mousa H, Dupont S, Déchanet-Merville J, Coumau-Gatbois E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115(11):3291–9.
Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030–8.
De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–71.
Kuijpers TW, IJspeert H, van Leeuwen EM, Jansen MH, Hazenberg MD, Weijer KC, et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG. Blood. 2011;117:5892–6.
Abraham RS, Recher M, Giliani S, Walter JE, Lee YN, Frugoni F, et al. Adult-onset manifestation of idiopathic T-cell lymphopenia due to a heterozygous RAG1 mutation. J Allergy Clin Immunol. 2013;131:1421–3.
Henderson LA, Frugoni F, Hopkins G, de Boer H, Pai SY, Lee YN, et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J Allergy Clin Immunol. 2013;132:969–71.
Abolhassani H, Wang N, Aghamohammadi A, Rezaei N, Lee YN, Frugoni F, et al. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:1375–80.
Kato T, Crestani E, Kamae C, Honma K, Yokosuka T, Ikegawa T, et al. RAG1 deficiency may present clinically as selective IgA deficiency. J Clin Immunol. 2015;35:280–8.
Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2014;133:1099–108.
IJspeert H, Driessen GJ, Moorhouse MJ, Hartwig NG, Wolska-Kusnierz B, Kalwak K, et al. Similar recombination-activating gene (RAG) mutations result in similar immunobiological effects but in different clinical phenotypes. J Allergy Clin Immunol. 2014;133:1124–33.
Smith C, Dutmer CM, Schmid DS, Bellini WJ, Gelfand EW, Asturias EJ. A toddler with rash, encephalopathy, and hemolytic anemia. J Pediatr Infect Dis. 2015. doi:10.1093/jpids/piv032.
Asai E, Wada T, Sakakibara Y, Toga A, Toma T, Shimizu T, et al. Analysis of mutations and recombination activity in RAG-deficient patients. Clin Immunol. 2011;138:172–7.
Sabry A, Hauk PJ, Jing H, Su HC, Stence NV, Mirsky DM, et al. Vaccine strain varicella-zoster virus-induced central nervous system vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol. 2014;133:1225–7.
Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, et al. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:522–7.
Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–38.
Kwan A, Puck JM. History and current status of newborn screening for severe combined immunodeficiency. Semin Perinatol. 2015;39:194–205.
Salt BH, Niemela JE, Pandey R, Hanson EP, Deering RP, Quinones R, et al. IKBKG (nuclear factor-kappa B essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J Allergy Clin Immunol. 2008;121(4):976–82.
Chen K, Wu W, Mathew D, Zhang Y, Browne SK, Rosen LB, et al. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations. J Allergy Clin Immunol. 2014;133:880–2.
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7.
Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–16.
Dowell SF, Bresee JS. Severe varicella associated with steroid use. Pediatrics. 1993;92:223–8.
Russell AF, Parrino J, Fisher Jr CL, Spieler W, Stek JE, Coll KE, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine. 2015;33:3129–34.
Leung J, Siegel S, Jones JF, Schulte C, Blog D, Schmid DS, et al. Fatal varicella due to the vaccine strain varicella-zoster virus. Hum Vaccin Immunother. 2014;10:146–9.
Schrauder A, Henke-Gendo C, Seidemann K, Sasse M, Cario G, Moericke A, et al. Varicella vaccination in a child with acute lymphoblastic leukaemia. Lancet. 2007;369:1232.
Woo EJ. Letter to the editor: fatal varicella due to the vaccine-strain varicella-zoster virus. Hum Vaccin Immunother. 2015;11:679.
Levy O, Orange JS, Hibberd P, Steinberg S, LaRussa P, Weinberg A, et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis. 2003;188:948–53.
Schuetz C, Neven B, Dvorak CC, Leroy S, Ege MJ, Pannicke U, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123:281–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of all Sources of Funding
C.D. was supported by a fellowship from Grifols. This work was partially supported by a grant from the National Institutes of Health (grant 5R01AI100887 to L.D.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Rights and permissions
About this article
Cite this article
Dutmer, C.M., Asturias, E.J., Smith, C. et al. Late Onset Hypomorphic RAG2 Deficiency Presentation with Fatal Vaccine-Strain VZV Infection. J Clin Immunol 35, 754–760 (2015). https://doi.org/10.1007/s10875-015-0207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0207-8