Abstract
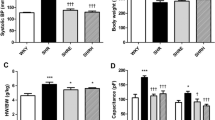
Hypertension is one of the major risk factors of cardiac hypertrophy and magnesium deficiency is suggested to be a contributing factor in the progression of this complication. In this study, we aimed to investigate the relationship between intracellular free Mg2+ levels and electrophysiological changes developed in the myocardium of L-NAME induced hypertensive rats. Hypertension was induced by administration of 40 mg/kg of L-NAME for 6 weeks, while magnesium treated rats fed with a diet supplemented with 1 g/kg of MgO for the same period. L-NAME administration for 6 weeks elicited a significant increase in blood pressure which was corrected with MgO treatment; thereby cardiac hypertrophy developing secondary to hypertension was prevented. Cytosolic free magnesium levels of ventricular myocytes were significantly decreased with hypertension and magnesium administration restored these changes. Hypertension significantly decreased the fractional shortening with slowing of shortening kinetics in left ventricular myocytes whereas magnesium treatment was capable of restoring hypertension-induced contractile dysfunction. Long-term magnesium treatment significantly restored the hypertension-induced prolongation in action potentials of ventricular myocytes and suppressed Ito and Iss currents. In contrast, hypertension dependent decrement in intracellular Mg2+ level did not cause a significant change in L-type Ca2+ currents, SR Ca2+ content and NCX activity. Nevertheless, hypertension mediated increase in superoxide anion, hydrogen peroxide and protein oxidation mitigated with magnesium treatment. In conclusion, magnesium administration improves mechanical abnormalities observed in hypertensive rat ventricular myocytes due to reduced oxidative stress. It is likely that, changes in intracellular magnesium balance may contribute to the pathophysiology of chronic heart diseases.








Similar content being viewed by others
References
Almulla HA, Bush PG, Steele MG, Flatman PW, Ellis D (2006) Sodium-dependent recovery of ionised magnesium concentration following magnesium load in rat heart myocytes. Pflugers Arch 451:657–667
Altura BM, Altura BT (1985) New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. I. Clinical aspects. Magnesium 4:226–244
Altura BM, Altura BT (1995) Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res 41:347–359
Aslan M, Canatan D (2008) Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp Hematol 36:1535–1544
Aydemir M, Ozturk N, Dogan S, Aslan M, Olgar Y, Ozdemir S (2012) Sodium tungstate administration ameliorated diabetes-induced electrical and contractile remodeling of rat heart without normalization of hyperglycemia. Biol Trace Elem Res 148:216–223
Bara M, Guiet-Bara A (1984) Potassium, magnesium and membranes. Review of present status and new findings. Magnesium 3:215–225
Brooksby P, Levi AJ, Jones JV (1993) The electrophysiological characteristics of hypertrophied ventricular myocytes from the spontaneously hypertensive rat. J Hypertens 11:611–622
Chan V, Fenning A, Levick SP, Loch D, Chunduri P, Iyer A, Teo YL, Hoey A, Wilson K, Burstow D, Brown L (2011) Cardiovascular changes during maturation and ageing in male and female spontaneously hypertensive rats. J Cardiovasc Pharmacol 57:469–478
Chen-Izu Y, Chen L, Banyasz T, McCulle SL, Norton B, Scharf SM, Agarwal A, Patwardhan A, Izu LT, Balke CW (2007) Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol 293:14
Chouabe C, Ricci E, Kurdi M, Legrand C, Bricca G, Bonvallet R (2009) Evaluation of remodeling in left and right ventricular myocytes from heterozygous (mRen2)27 transgenic rats. Gen Physiol Biophys 28:24–38
De Artinano AA, Gonzalez VL (1999) Endothelial dysfunction and hypertensive vasoconstriction. Pharmacol Res 40:113–124
DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schonbein GW (2006) Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation 13:551–566
Doggrell SA, Nand V, Henderson CJ (1999) The effects of lignocaine and tetrodotoxin on the action potentials and contractions of left ventricles from normo- and hypertensive rats. Gen Pharmacol 32:429–437
Fatholahi M, LaNoue K, Romani A, Scarpa A (2000) Relationship between total and free cellular Mg(2+) during metabolic stimulation of rat cardiac myocytes and perfused hearts. Arch Biochem Biophys 374:395–401
Gibbons GH (1997) Endothelial function as a determinant of vascular function and structure: a new therapeutic target. Am J Cardiol 79:3–8
Griffiths EJ (2000) Calcium handling and cell contraction in rat cardiomyocytes depleted of intracellular magnesium. Cardiovasc Res 47:116–123
Grubbs RD, Maguire ME (1987) Magnesium as a regulatory cation: criteria and evaluation. Magnesium 6:113–127
Gupta RK, Moore RD (1980) 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem 255:3987–3993
Gupta RK, Benovic JL, Rose ZB (1978) Magnetic resonance studies of the binding of ATP and cations to human hemoglobin. J Biol Chem 253:6165–6171
Gusev K, Niggli E (2008) Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J Gen Physiol 132:721–730
Ishiguro S, Matsuyama T, Sakaguchi H, Nishio A (1997) Ex vivo study of the increased sensitivity to NO of endothelium-denuded thoracic aortas isolated from dietary magnesium-deficient rats. Magnes Res 10:21–31
Keung EC (1989) Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res 64:753–763
Kharb S, Singh V (2000) Magnesium deficiency potentiates free radical production associated with myocardial infarction. J Assoc Physicians India 48:484–485
Kirschenlohr HL, Metcalfe JC, Morris PG, Rodrigo GC, Smith GA (1988) Ca2+ transient, Mg2+, and pH measurements in the cardiac cycle by 19F NMR. Proc Natl Acad Sci U S A 85:9017–9021
Kiyosue T (2002) Removal of intracellular Mg(2+) activates cardiac Na(+)/Ca(2+) exchanger. Cardiovasc Res Feb 1;53(2):290–1.
Kramer JH, Misik V, Weglicki WB (1994) Magnesium-deficiency potentiates free radical production associated with postischemic injury to rat hearts: vitamin E affords protection. Free Radic Biol Med 16:713–723
Lacy F, Gough DA, Schmid-Schonbein GW (1998) Role of xanthine oxidase in hydrogen peroxide production. Free Radic Biol Med 25:720–727
Laurant P, Touyz RM, Schiffrin EL (1997) Effect of magnesium on vascular tone and reactivity in pressurized mesenteric resistance arteries from spontaneously hypertensive rats. Can J Physiol Pharmacol 75:293–300
Li X, Jiang W (2000) Electrical remodeling of membrane ionic channels of hypertrophied ventricular myocytes from spontaneously hypertensive rats. Chin Med J 113:584–587
Li L, Desantiago J, Chu G, Kranias EG, Bers DM (2000) Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278:H769–H779
Li SY, Golden KL, Jiang Y, Wang GJ, Privratsky JR, Zhang X, Eason AR, Culver B, Ren J (2005) Inhibition of sarco(endo)plasmic reticulum Ca2 + −ATPase differentially regulates contractile function in cardiac myocytes from normotensive and spontaneously hypertensive rats: role of Ca2+ regulatory proteins. Cell Biochem Biophys 42:1–12
Liao F, Folsom AR, Brancati FL (1998) Is low magnesium concentration a risk factor for coronary heart disease? The atherosclerosis risk in communities (ARIC) study. Am Heart J 136:480–490
Luo J, Xuan YT, Gu Y, Prabhu SD (2006) Prolonged oxidative stress inverts the cardiac force-frequency relation: role of altered calcium handling and myofilament calcium responsiveness. J Mol Cell Cardiol 40:64–75
Mahboob T, Mumtaz M, Haleem MA (1996) Electrolyte content of serum, erythrocyte, kidney and heart tissue in salt induced hypertensive rats. Life Sci 59:731–737
Manju L, Nair RR (2006) Magnesium deficiency augments myocardial response to reactive oxygen species. Can J Physiol Pharmacol 84:617–624
Mertens MJ, Mathy MJ, Pfaffendorf M, van Zwieten PA (1992) Depressed inotropic response to beta-adrenoceptor agonists in the presence of advanced cardiac hypertrophy in hearts from rats with induced aortic stenosis and from spontaneously hypertensive rats. J Hypertens 10:1361–1368
Michailova AP, Belik ME, McCulloch AD (2004) Effects of magnesium on cardiac excitation-contraction coupling. J Am Coll Nutr 23:514S–517S
Milnes JT, MacLeod KT (2001) Reduced ryanodine receptor to dihydropyridine receptor ratio may underlie slowed contraction in a rabbit model of left ventricular cardiac hypertrophy. J Mol Cell Cardiol 33:473–485
Morton JJ, Beattie EC, Speirs A, Gulliver F (1993) Persistent hypertension following inhibition of nitric oxide formation in the young Wistar rat: role of renin and vascular hypertrophy. J Hypertens 11:1083–1088
Ozturk N, Yaras N, Ozmen A, Ozdemir S (2013) Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr 45:343–352
Paravicini TM, Touyz RM (2008) NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31: dc08–s247
Pollock DM (1999) Chronic studies on the interaction between nitric oxide and endothelin in cardiovascular and renal function. Clin Exp Pharmacol Physiol 26:258–261
Prohaska JR (1991) Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121:355–363
Raju B, Murphy E, Levy LA, Hall RD, London RE (1989) A fluorescent indicator for measuring cytosolic free magnesium. Am J Phys 256:C540–C548
Ren J (2007) Influence of gender on oxidative stress, lipid peroxidation, protein damage and apoptosis in hearts and brains from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 34:432–438
Sharikabad MN, Ostbye KM, Lyberg T, Brors O (2001) Effect of extracellular Mg(2+) on ROS and Ca(2+) accumulation during reoxygenation of rat cardiomyocytes. Am J Physiol Heart Circ Physiol 280:H344–H353
Sonoyama K, Igawa O, Miake J, Yamamoto Y, Sugihara S, Sasaki N, Shimoyama M, Hamada T, Taniguchi S, Yoshida A, Ogino K, Shigemasa C, Hoshikawa Y, Kurata Y, Shiota G, Narahashi T, Horiuchi M, Matsubara H, Ninomiya H, Hisatome I (2005) Effects of angiotensin II on the action potential durations of atrial myocytes in hypertensive rats. Hypertens Res 28:173–179
Sontia B, Touyz RM (2007) Role of magnesium in hypertension. Arch Biochem Biophys 458:33–39
Sumandea MP, Steinberg SF (2011) Redox signaling and cardiac sarcomeres. J Biol Chem 286:9921–9927
Tashiro M, Inoue H, Konishi M (2014) Physiological pathway of magnesium influx in rat ventricular myocytes. Biophys J 107:2049–2058
Touyz RM (2008) Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 294:11
Wang M, Berlin JR (2007) Voltage-dependent modulation of L-type calcium currents by intracellular magnesium in rat ventricular myocytes. Arch Biochem Biophys 458:65–72
Wang M, Tashiro M, Berlin JR (2004) Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J Physiol 555:383–396
Wang L, Lopaschuk GD, Clanachan AS (2008) H(2)O(2)-induced left ventricular dysfunction in isolated working rat hearts is independent of calcium accumulation. J Mol Cell Cardiol 45:787–795
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104:79–86
Zatz R, Baylis C (1998) Chronic nitric oxide inhibition model 6 years on. Hypertension 32:958–964
Acknowledgments
This study was supported by a grant from Akdeniz University Scientific Research Coordination Unit, Turkey (2011.03.0122.006). This study was carried out as PhD thesis by N. Ozturk presented to Akdeniz University Health Sciences Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Ozturk, N., Olgar, Y., Aslan, M. et al. Effects of magnesium supplementation on electrophysiological remodeling of cardiac myocytes in L-NAME induced hypertensive rats. J Bioenerg Biomembr 48, 425–436 (2016). https://doi.org/10.1007/s10863-016-9666-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-016-9666-8