Abstract
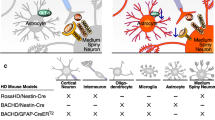
Preferential striatal neurodegeneration is a hallmark of Huntington’s disease (HD) pathogenesis, which has been associated with mitochondrial dysfunction. Evidence from genetic HD models suggest that mutant huntingtin (mHtt) compromises mitochondrial bioenergetics and dynamics, preventing efficient calcium handling and ATP generation in neuronal networks. Striatal neurons receive abundant glutamatergic input from the cortex, forming tripartite synapses with astrocytic partners. These are involved in bidirectional communication, play neuroprotective roles, and emerging evidence suggests that astrocyte dysfunction supports non-cell autonomous neurodegeneration. In addition to mHtt effects, inherent mitochondria vulnerability within striatal neurons and astrocytes may contribute for preferential neurodegeneration in HD. Dysfunctional astrocytic mitochondria in cortico-striatal tripartite synapses might be particularly relevant in the pathogenesis of juvenile/infantile HD, frequently associated with seizures and abnormally large mHtt polyglutamine expansions. This review discusses our work, primarily addressing in situ mitochondrial function in neurons and astrocytes, in the context of related work within the HD-mitochondria field.
Similar content being viewed by others
References
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215
Bambrick L, Kristian T, Fiskum G (2004) Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res 29:601–608
Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G (2006) Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr 38:43–47
Boitier E, Rea R, Duchen MR (1999) Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol 145:795–808
Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S (2009) Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A 106:22480–22485
Bradford J, Shin JY, Roberts M, Wang CE, Sheng G, Li S, Li XJ (2010) Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem. doi:10.1074/jbc.M109.083287
Brouillet E, Jacquard C, Bizat N, Blum D (2005) 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J Neurochem 95:1521–1540
Brown MR, Sullivan PG, Geddes JW (2006) Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem 281:11658–11668
Browne SE (2008) Mitochondria and Huntington’s disease pathogenesis: insight from genetic and chemical models. Ann N Y Acad Sci 1147:358–382
Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM (2003) Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci 23:4858–4867
Brustovetsky N, LaFrance R, Purl KJ, Brustovetsky T, Keene CD, Low WC, Dubinsky JM (2005) Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of Huntington’s Disease. J Neurochem 93:1361–1370
Calabresi P, Centonze D, Bernardi G (2000) Cellular factors controlling neuronal vulnerability in the brain: a lesson from the striatum. Neurology 55:1249–1255
Chalmers S, Nicholls DG (2003) The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278:19062–19070
Chang DT, Reynolds IJ (2006) Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol 80:241–268
Chang DT, Rintoul GL, Pandipati S, Reynolds IJ (2006) Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis 22:388–400
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18:R169–R176
Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M (2004) Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13:1407–1420
Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT (2009) Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 32:591–601
Dienel GA, Hertz L (2005) Astrocytic contributions to bioenergetics of cerebral ischemia. Glia 50:362–388
Djousse L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH (2002) Weight loss in early stage of Huntington’s disease. Neurology 59:1325–1330
Domingues A, Almeida S, da Cruz e Silva EF, Oliveira CR, Rego AC (2007) Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochem Int 50:872–880
Gines S, Ivanova E, Seong IS, Saura CA, MacDonald ME (2003) Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington’s disease knock-in striatal cells. J Biol Chem 278:50514–50522
Goebel HH, Heipertz R, Scholz W, Iqbal K, Tellez-Nagel I (1978) Juvenile Huntington chorea: clinical, ultrastructural, and biochemical studies. Neurology 28:23–31
Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH (1996) Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol 39:385–389
Gusella JF, MacDonald ME (2000) Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci 1:109–115
Hsiao HY, Chern Y (2010) Targeting glial cells to elucidate the pathogenesis of Huntington’s disease. Mol Neurobiol. doi:10.1007/s12035-009-8097-5
Ilieva H, Polymenidou M, Cleveland DW (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187:761–772
Jekabsons MB, Nicholls DG (2004) In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J Biol Chem 279:32989–33000
Kann O, Kovacs R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292:C641–C657
Kazantsev AG, Thompson LM (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7:854–868
Klohn PC, Soriano ME, Irwin W, Penzo D, Scorrano L, Bitsch A, Neumann HG, Bernardi P (2003) Early resistance to cell death and to onset of the mitochondrial permeability transition during hepatocarcinogenesis with 2-acetylaminofluorene. Proc Natl Acad Sci U S A 100:10014–10019
Leventhal L, Sortwell CE, Hanbury R, Collier TJ, Kordower JH, Palfi S (2000) Cyclosporin A protects striatal neurons in vitro and in vivo from 3-nitropropionic acid toxicity. J Comp Neurol 425:471–478
Lievens JC, Birman S (2007) Astrocytes in Huntington’s chorea: accomplice or guilty of the neuronal death? Med Sci (Paris) 23:845–849
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E (2009) Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ 16:899–909
Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10:1355–1360
Monti B, Polazzi E, Contestabile A (2009) Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol 2:95–109
Oliveira JM (2010a) Nature and cause of mitochondrial dysfunction in Huntington’s disease: focusing on Huntingtin and the Striatum. J Neurochem doi:10.1111/j.1471-4159.2010.06741.x
Oliveira JM (2010b) Techniques to investigate neuronal mitochondrial function and its pharmacological modulation. Curr Drug Targets. Accepted for publication
Oliveira JM, Gonçalves J (2009a) In situ mitochondrial Ca2+ buffering differences of intact neurons and astrocytes from cortex and striatum. J Biol Chem 284:5010–5020
Oliveira JM, Gonçalves J (2009b) Neuronal and astrocytic mitochondrial dynamics as vulnerability factors and targets for neuroprotection [Abstract]. Frontiers Neurosci. doi:10.3389/conf.neuro.01.2009.11.041
Oliveira JM, Almeida S, Domingues A, Ellerby LM, Gonçalves J, Oliveira CR, Rego AC (2004) Calcium mobilising pathways and Δψm in striatal progenitor cells [Abstract]. Fund Clin Pharmacol 18(suppl 1):P19.36. doi:10.1111/j.1472-8206.2004.00260.x
Oliveira JM, Chen S, Almeida S, Riley R, Gonçalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC (2006) Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci 26:11174–11186
Oliveira JM, Jekabsons MB, Chen S, Lin A, Rego AC, Gonçalves J, Ellerby LM, Nicholls DG (2007) Mitochondrial dysfunction in Huntington’s disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. J Neurochem 101:241–249
Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ (2008) N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci 28:2783–2792
Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT (2002) Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci 5:731–736
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431
Reyes RC, Parpura V (2008) Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci 28:9682–9691
Reynolds IJ, Malaiyandi LM, Coash M, Rintoul GL (2004) Mitochondrial trafficking in neurons: a key variable in neurodegeneration? J Bioenerg Biomembr 36:283–286
Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ (2003) Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci 23:7881–7888
Rossi DJ, Brady JD, Mohr C (2007) Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 10:1377–1386
Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ (2005) Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol 171:1001–1012
Squitieri F, Frati L, Ciarmiello A, Lastoria S, Quarrell O (2006) Juvenile Huntington’s disease: does a dosage-effect pathogenic mechanism differ from the classical adult disease? Mech Ageing Dev 127:208–212
Tobin AJ, Signer ER (2000) Huntington’s disease: the challenge for cell biologists. Trends Cell Biol 10:531–536
Trushina E et al (2004) Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol 24:8195–8209
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777:1092–1097
Wang H, Lim PJ, Karbowski M, Monteiro MJ (2009a) Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet 18:737–752
Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X (2009b) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem 109(Suppl 1):153–159
Author information
Authors and Affiliations
Corresponding author
Additional information
Journal of Bioenergetics & Biomembranes Mini-Review Series on “Mitochondrial Matters of the Brain: Role(s) in Huntington’s Disease”. Ed. P. L. Pedersen
Rights and permissions
About this article
Cite this article
Oliveira, J.M.A. Mitochondrial bioenergetics and dynamics in Huntington’s disease: tripartite synapses and selective striatal degeneration. J Bioenerg Biomembr 42, 227–234 (2010). https://doi.org/10.1007/s10863-010-9287-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-010-9287-6