Abstract
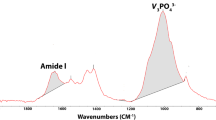
Bone autografts are often used for reconstruction of bone defects; however, due to the limitations of autografts, researchers have been in search of bone substitutes. Dentin is of particular interest for this purpose due to high similarity to bone. This in vitro study sought to assess the surface characteristics and biological properties of dentin samples prepared with different treatments. This study was conducted on regular (RD), demineralized (DemD), and deproteinized (DepD) dentin samples. X-ray diffraction and Fourier transform infrared spectroscopy were used for surface characterization. Samples were immersed in simulated body fluid, and their bioactivity was evaluated under a scanning electron microscope. The methyl thiazol tetrazolium assay, scanning electron microscope analysis and quantitative real-time polymerase chain reaction were performed, respectively to assess viability/proliferation, adhesion/morphology and osteoblast differentiation of cultured human dental pulp stem cells on dentin powders. Of the three dentin samples, DepD showed the highest and RD showed the lowest rate of formation and deposition of hydroxyapatite crystals. Although, the difference in superficial apatite was not significant among samples, functional groups on the surface, however, were more distinct on DepD. At four weeks, hydroxyapatite deposits were noted as needle-shaped accumulations on DemD sample and numerous hexagonal HA deposit masses were seen, covering the surface of DepD. The methyl thiazol tetrazolium, scanning electron microscope, and quantitative real-time polymerase chain reaction analyses during the 10-day cell culture on dentin powders showed the highest cell adhesion and viability and rapid differentiation in DepD. Based on the parameters evaluated in this in vitro study, DepD showed high rate of formation/deposition of hydroxyapatite crystals and adhesion/viability/osteogenic differentiation of human dental pulp stem cells, which may support its osteoinductive/osteoconductive potential for bone regeneration.




Similar content being viewed by others
References
Tabatabaei FS, Motamedian SR, Khosraviani K, Gholipour F, Khojasteh A. Craniomaxillofacial bone engineering by scaffolds loaded with stem cells: a systematic review. J Dent Sch. 2012;30(2):113–30.
Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38:S3–S6.
Brydone A, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224(12):1329–43.
Roberts-Clark D, Smith A. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45(11):1013–6.
Murata M. Bone engineering using human demineralized dentin matrix and recombinant human BMP-2. J Hard Tissue Biol. 2005;14(2):80–1.
Gomes MF, Banzi ECdF, Destro MFdSS, Lavinicki V, Goulart MdGV. Homogenous demineralized dentin matrix for application in cranioplasty of rabbits with alloxan-induced diabetes: histomorphometric analysis. Int J Oral Maxillofac Implants. 2007;22(6):939–47.
Kim Y-K. Bone graft material using teeth. J Korean Assoc Oral Maxillofac Surg. 2012;38(3):134–8.
Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81(9):1264–72.
Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1):18
Murata M, Akazawa T, Mitsugi M, Um I-W, Kim K-W, Kim Y-K. Human dentin as novel biomaterial for bone regeneration. In: Pignatello R, editor.: InTech; 2011. pp. 127–40.
Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998;24(3):124–32.
Yeomans J, Urist M. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12(8):999–IN16.
Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation by osteoinduction in rats. Scand J Dent Res. 1990;98(5):434–41.
Tabatabaei FS, Ai J, Kashi TSJ, Khazaei M, Kajbafzadeh A-M, Ghanbari Z. Effect of dentine matrix proteins on human endometrial adult stem-like cells: in vitro regeneration of odontoblasts cells. Arch Oral Biol. 2013;58(7):871–9.
Gu H-r, Jang H-s, Kim S-w, Park J-c. Periodontal regeneration using the mixture of human tooth-ash and plaster of paris in dogs. J Periodontol. 2006;36(1).
Moharamzadeh K, Freeman C, Blackwood K. Processed bovine dentine as a bone substitute. Br J Oral Maxillofac Surg. 2008;46(2):110–3.
SoYoungChun B, HyoJungLee T, SeHeangOh J, HongInShin E. Composite scaffold with demineralized dentin particle and poly (lactic co-glycolic acid) for cranial bone regeneration. Tissue Eng Regen Med. 2011;8(3):306–13.
Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32(20):4525–38.
Shah FA, Brauer DS, Wilson RM, Hill RG, Hing KA. Influence of cell culture medium composition on in vitro dissolution behavior of a fluoride-containing bioactive glass. J Biomed Mater Res Part A. 2014;102A:647–54.
Tabatabaei FS, Jazayeri M, Ghahari P, Haghighipour N. Effects of equiaxial strain on the differentiation of dental pulp stem cells without using biochemical reagents. Mol Cell Biomech. 2014;11(3):209–20.
Tabatabaei FS, Torshabi M, Nasab MM, Khosraviani K, Khojasteh A. Effect of low-level diode laser on proliferation and osteogenic differentiation of dental pulp stem cells. Laser Phys. 2015;25(9):095602.
Smith A, Scheven B, Takahashi Y, Ferracane J, Shelton R, Cooper P. Dentine as a bioactive extracellular matrix. Arch Oral Biol. 2012;57(2):109–21.
Tabatabaei FS, Tatari S, Samadi R, Moharamzadeh K. 2016. Different methods of dentin processing for application in bone tissue engineering: A systematic review. J Biomed Mater Res Part A 2016:104A:2616–2627.
Pappalardo S, Mastrangelo F, Reale MD, Cappello V, Ciampoli C, Carlino V, et al. Bone regeneration: in vitro evaluation of the behaviour of osteoblast-like MG63 cells placed in contact with polylactic-co-glycolic acid, deproteinized bovine bone and demineralized freeze-dried bone allograft. J Biol Regul Homeost Agents. 2007;22(3):175–83.
Fugazzotto P, De Paoli S, Benfenati S. The use of allogenic freeze-dried dentin in the repair of periodontal osseous defects in humans. Quintessence Int. 1986;17(8):461.
Şen BH, Ertürk Ö, Pişkin B. The effect of different concentrations of EDTA on instrumented root canal walls. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(4):622–7.
Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13(01):94–117.
Shi D. Introduction to biomaterials. Beijing: Tsinghua University Press; 2006.
Chen Po-Yu, Toroian D, Price PA, McKittrick J. Minerals form a continuum phase in mature cancellous bone. Calcif Tissue Int. 2011;88(5):351–61.
Greenspan DC. Comparison of a synthetic and bovine derived hydroxyapatite bone graft substitute. Zimmer Dental Inc. 2012:1–4.
Toledano M, Osorio E, Cabello I, Osorio, R. Early dentine remineralisation: Morpho-mechanical assessment. J Dent. 42(4):384–94.
Akazawa T, Murata M, Hino J, Nagano F, Shigyo T, Nomura T, et al. Surface structure and biocompatibility of demineralized dentin matrix granules soaked in a simulated body fluid. Appl Surf Sci. 2012;262:51–5.
Kim Y-K, Kim S-G, Byeon J-H, Lee H-J, Um I-U, Lim S-C, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):496–503.
Kim H-K, Jang J-W, Lee C-H. Surface modification of implant materials and its effect on attachment and proliferation of bone cells. J Mater Sci Mater Med. 2004;15(7):825–30.
Kim Y-K, Kim S-G, Yun P-Y, Yeo I-S, Jin S-C, Oh J-S, et al. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2014;117(1):e39–45.
Elkayar A, Elshazly Y, Assaad M. Properties of hydroxyapatite from bovine teeth. Bone Tissue Regen Insights. 2009;2:31–6.
Pappalardo S, Carlino V, Brutto D, Sinatra F. How do biomaterials affect the biological activities and responses of cells? An in vitro study. Minerva stomatologica. 2010;59(9):445–64.
Xiao L, Tsutsui T. Characterization of human dental pulp cells‐derived spheroids in serum‐free medium: stem cells in the core. J Cell Biochem. 2013;114(11):2624–36.
Chun SY, Acharya B, Lee HJ, Kwon T-g, Oh SH, Lee JH, et al. Composite scaffold with demineralized dentin particle and poly(lactic co- glycolic acid) for cranial bone regeneration. Tissue Eng Regen Med. 2011;8(3):306–13.
Tabatabaei FS, Torshabi M. Effects of non-collagenous proteins, TGF-β1, and PDGF-BB on viability and proliferation of dental pulp stem cells. J Oral Maxillofac Res. 2016;7(1):e4.
Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30(35):6708–23.
Pietrzak WS, Ali SN, Chitturi D, Jacob M, Woodell-May JE. BMP depletion occurs during prolonged acid demineralization of bone: characterization and implications for graft preparation. Cell Tissue Bank. 2011;12(2):81–8.
Smith AJ. Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ. 2003;67(6):678–89.
Smith AJ, Patel M, Graham L, Sloan AJ, Cooper PR. Dentine regeneration: key roles for stem cells and molecular signalling. Oral Biosci Med. 2005;2:127–32.
Galler K, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer P, et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J. 2015.
Miron R, Zhang Y. Osteoinduction a review of old concepts with new standards. J Dent Res. 2012;91(8):736–44.
Roberts SJ, Chen Y, Moesen M, Schrooten J, Luyten FP. Enhancement of osteogenic gene expression for the differentiation of human periosteal derived cells. Stem Cell Res. 2011;7(2):137–44.
Acknowledgments
This paper was derived from a doctorate thesis, supported by the School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical sciences (Project No. 1345) and approved by the ethical committee (No. 1345-16915) of this university.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tabatabaei, F., Tatari, S., Samadi, R. et al. Surface characterization and biological properties of regular dentin, demineralized dentin, and deproteinized dentin. J Mater Sci: Mater Med 27, 164 (2016). https://doi.org/10.1007/s10856-016-5780-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5780-8