Abstract
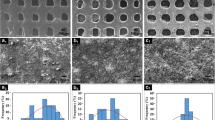
Three-dimensional printer (3DP) (Z-Corp) is a solid freeform fabrication system capable of generating sub-millimeter physical features required for tissue engineering scaffolds. By using plaster composite materials, 3DP can fabricate a universal porogen which can be injected with a wide range of high melting temperature biomaterials. Here we report results toward the manufacture of either pure polycaprolactone (PCL) or homogeneous composites of 90/10 or 80/20 (w/w) PCL/beta-tricalcium phosphate (β-TCP) by injection molding into plaster composite porogens fabricated by 3DP. The resolution of printed plaster porogens and produced scaffolds was studied by scanning electron microscopy. Cytotoxicity test on scaffold extracts and biocompatibility test on the scaffolds as a matrix supporting murine osteoblast (7F2) and endothelial hybridoma (EAhy 926) cells growth for up to 4 days showed that the porogens removal process had only negligible effects on cell proliferation. The biodegradation tests of pure PCL and PCL/β-TCP composites were performed in DMEM with 10 % (v/v) FBS for up to 6 weeks. The PCL/β-TCP composites show faster degradation rate than that of pure PCL due to the addition of β-TCP, and the strength of 80/20 PCL/β-TCP composite is still suitable for human cancellous bone healing support after 6 weeks degradation. Combining precisely controlled porogen fabrication structure, good biocompatibility, and suitable mechanical properties after biodegradation, PCL/β-TCP scaffolds fabricated by 3DP porogen method provide essential capability for bone tissue engineering.









Similar content being viewed by others
References
Williams D. Benefit and risk in tissue engineering. Mater Today. 2004;7:24–9.
Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19.
Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–59.
Thomson RC, Mikos AG, Beahm E, Lemon JC, Satterfield WC, Aufdemorte TB, et al. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials. 1999;20:2007–18.
Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98.
Zhang QW, Mochalin VN, Neitzel I, Knoke IY, Han JJ, Klug CA, et al. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials. 2011;32:87–94.
Neumann M, Epple M. Composites of calcium phosphate and polymers as bone substitution materials. Eur J Trauma. 2006;32:125–31.
Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10:33–41.
Rohner D, Hutmacher DW, Cheng TK, Oberholzer M, Hammer B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66B:574–80.
Ruhe PQ, Hedberg EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(dl-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85A:75–81.
Xu HHK, Quinn JB, Takagi S, Chow LC. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials. 2004;25:1029–37.
Barralet JE, Grover L, Gaunt T, Wright AJ, Gibson IR. Preparation of macroporous calcium phosphate cement tissue engineering scaffold. Biomaterials. 2002;23:3063–72.
Yang Q, Chen L, Shen X, Tan Z. Preparation of polycaprolactone tissue engineering scaffolds by improved solvent casting/particulate leaching method. J Macromol Sci Part B Phys. 2006;45:1171–81.
Heijkants R, Van Tienen TG, De Groot JH, Pennings AJ, Buma P, Veth RPH, et al. Preparation of a polyurethane scaffold for tissue engineering made by a combination of salt leaching and freeze-drying of dioxane. J Mater Sci. 2006;41:2423–8.
Mikos (AGH, TX), Sarakinos, Georgios (Boston, MA), Vacanti, Joseph P. (Winchester, MA), Langer, Robert S. (Newton, MA), Cima, Linda G. (Lexington, MA). Biocompatible polymer membranes and methods of preparation of three-dimensional membrane structures. United States: Massachusetts Institute of Technology (Cambridge, MA), Children’s Medical Center Corporation (Boston, MA); 1996.
Fukuhira Y, Kitazono E, Hayashi T, Kaneko H, Tanaka M, Shimomura M, et al. Biodegradable honeycomb-patterned film composed of poly(lactic acid) and dioleoylphosphatidylethanolamine. Biomaterials. 2006;27:1797–802.
Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng Trans ASME. 1991;113:143–51.
Zhang W, Yao D, Zhang Q, Zhou JG, Lelkes PI. Fabrication of interconnected microporous biomaterials with high hydroxyapatite nanoparticle loading. Biofabrication. 2010;2.
Manjubala I, Woesz A, Pilz C, Rumpler M, Fratzl-Zelman N, Roschger P, et al. Biomimetic mineral-organic composite scaffolds with controlled internal architecture. J Mater Sci Mater Med. 2005;16:1111–9.
Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–103.
Roy TD, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J Biomed Mater Res A. 2003;66A:283–91.
Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer–ceramic scaffolds. Biomaterials. 2003;24:181–94.
Lu L, Zhang Q, Wootton D, Lelkes PI, Zhou J. A novel sucrose porogen-based solid freeform fabrication system for bone scaffold manufacturing. Rapid Prototyping J. 2010;16:365–76.
Mooney DJ, Baldwin DF, Suh NP, Vacanti LP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22.
Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VKC, Wootton DM, Lelkes PI, et al. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering. Biomaterials. 2006;27:4399–408.
Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137–46.
Geissler U, Hempel U, Wolf C, Scharnweber D, Worch H, Wenzel KW. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J Biomed Mater Res. 2000;51:752–60.
Andrianarivo AG, Robinson JA, Mann KG, Tracy RP. Growth on type-I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells. J Cell Physiol. 1992;153:256–65.
Schantz JT, Hutmacher DW, Ng KW, Khor HL, Lim TC, Teoh SH. Evaluation of a tissue-engineered membrane-cell construct for guided bone regeneration. Int J Oral Maxillofac Implants. 2002;17:161–74.
Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. The Intracellular degradation of poly(epsilon-caprolactone). J Biomed Mater Res. 1985;19:437–44.
Ali SAM, Zhong SP, Doherty PJ, Williams DF. Mechanisms of polymer degradation in implantable devices. 1. Poly(caprolactone). Biomaterials. 1993;14:648–56.
Kim HW, Knowles JC, Kim HE. Development of hydroxyapatite bone scaffold for controlled drug release via poly(epsilon-caprolactone) and hydroxyapatite hybrid coatings. J Biomed Mater Res B Appl Biomater. 2004;70B:240–9.
Timmer MD, Ambrose CG, Mikos AG. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials. 2003;24:571–7.
Peter SJ, Nolley JA, Widmer MS, Merwin JE, Yaszemski MJ, Yasko AW, et al. In vitro degradation of a poly(propylene fumarate)/beta-tricalcium phosphate composite orthopaedic scaffold. Tissue Eng. 1997;3:207–15.
Looney MA, Park JB. Molecular and mechanical property changes during aging of bone cement in vitro and in vivo. J Biomed Mater Res. 1986;20:555–63.
Misch CE, Qu ZM, Bidez MW. Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and. Surgical placement. J Oral Maxillofac Surg. 1999;57:700–6.
Acknowledgments
We gratefully thank National Science Foundation (NSF) for its financial support (DMI–0300405, CMMI-0700139 and CMMI-0925348). Additionally, the authors are grateful to Dr. Wei Sun for providing access to 3D printer for this study. We also would like to thank the laboratory of Dr. Giuseppe Palmese for assistance with GPC degradation tests and the laboratory of Dr. Boris Polyak for providing the access to plate reader. The Centralized Research Facility (CRF) of the College of Engineering, Drexel University provided access to electron microscopes used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Zhang, Q., Wootton, D. et al. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J Mater Sci: Mater Med 23, 2217–2226 (2012). https://doi.org/10.1007/s10856-012-4695-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4695-2