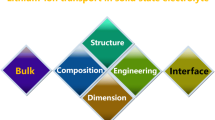
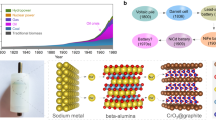
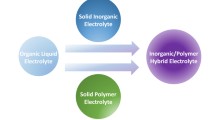
Abstract
All-solid-state batteries are swiftly gaining the attention of the research community owing to their widespread applications in electric vehicles, digital electronics, portable appliances, etc. A battery comprises three components: cathode, anode and electrolyte. An electrolyte is the heart of the battery and plays a crucial role in the overall performance of the battery. In order to make the review more focused, all-solid-state Li-ion batteries (ASSLIBs) have been considered. This review covers the architecture of ASSLIBs, advantages, and characteristics of the solid polymer electrolytes. The important preparation methods are summarized, followed by the characterizations for testing the suitability of electrolytes for solid-state batteries. The discussion is focused on the ‘state of the art’ in the field of solid-state batteries, device fabrication, and comparison in terms of capacity, energy density, and cyclic stability. In the last section, the ion conduction mechanism in different solid polymer electrolytes is discussed. Finally, it is tried to give a possible outlook for developing future hybrid and multifunctional electrolytes which can act as a bridge for developing solid-state batteries covering a broad range of applications.

(Reproduced with permission from Ref. [11] © RSC Publishing 2017)

(Reproduced with permission from Ref. [32] © Elsevier 2018)

[Reproduced with permission from Ref. [33] © Electrochemical Society 2017]


(Reproduced with permission from Ref. [39] © AAAS 2018)

(Reproduced with permission from Ref. [38] © MDPI 2018)

(Reproduced with permission from Ref. [63], © AAAS 2018)



(Reproduced with permission from Ref. [114] © Elsevier 2016)

(Reproduced with permission from Ref. [117] © American Chemical Society 2016)

(Reproduced with permission from Ref. [101] © American Chemical Society 2012)

(Reproduced with permission from Ref. [118] © Elsevier 2019)

(Reproduced with permission from Ref. [119] © Elsevier 2019)

(Reproduced with permission from Ref. [120] © The Electrochemical Society 2019)


(Reproduced with permission from Ref. [124] © Elsevier 2018)

(Reproduced with permission from Ref. [130] © Elsevier 2018)

(Reproduced with permission from Ref. [113] © American Chemical Society 2018)

(Reproduced with permission from Ref. [135] © Wiley 2018)

(Reproduced with permission from Ref. [137] © RSC Publishing 2018)

(Reproduced with permission from Ref. [141] © Elsevier 2018)

(Reproduced with permission from Ref. [144] © Elsevier 2018)

(Reproduced with permission from Ref. [92] © Elsevier 2016)

(Reproduced with permission from Ref. [147] © Elsevier 2008)

(Reproduced with permission from Ref. [154], © Elsevier 2017)

(Reproduced with permission from Ref. [157], © American Chemical Society 2018)

(Reproduced with permission from Ref. [160] © The Royal Society of Chemistry 2019)

(Reproduced with permission from Ref. [162] © 2016)

(Reproduced with permission from Ref. [164] © Elsevier 2019)

(Reproduced with permission from Ref. [166] © American Chemical Society 2018)

(Reproduced with permission from Ref. [167] © John Wiley and Sons 2015)

(Reproduced with permission from Ref. [21] © Royal Society of Chemistry 2015)

(Reproduced with permission from Ref. [169] © John Wiley and Sons 2015)

(Reproduced with permission from Ref. [117] © American Chemical Society 2016)

(Reproduced with permission from Ref. [188] © John Wiley and Sons 2019)
Similar content being viewed by others
References
Arya A, Sharma AL (2017) Insights into the use of polyethylene oxide in energy storage/conversion devices: a critical review. J Phys D Appl Phys 50(44):443002
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394(6692):456–458
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J Phys D Appl Phys 41(22):223001
Armand M, Tarascon JM (2008) Building better batteries. Nature 451(7179):652–657
Tarascon JM, Armand M (2011) Issues and challenges facing rechargeable lithium batteries. In: Materials for sustainable energy: a collection of peer-reviewed research and review articles from Nature Publishing Group, pp 171–179
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104(10):4271–4302
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195(9):2419–2430
Gulzar U, Goriparti S, Miele E, Li T, Maidecchi G, Toma A, De Angelis F, Capiglia C, Zaccaria RP (2016) Next-generation textiles: from embedded supercapacitors to lithium ion batteries. J Mater Chem A 4(43):16771–16800
Goodenough JB, Kim Y (2011) Challenges for rechargeable batteries. J Power Sources 22(3):587–603
Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D (2011) A review of advanced and practical lithium battery materials. J Mater Chem 21(27):9938–9954
Wu F, Yushin G (2017) Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ Sci 10(2):435–459
Croy JR, Abouimrane A, Zhang Z (2014) Next-generation lithium-ion batteries: the promise of near-term advancements, MRS Bulletin. MRS Bull 39(5):407–415
Thackeray MM, Wolverton C, Isaacs ED (2012) Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ Sci 5(7):7854–7863
Meyers RA (ed) (2012) Encyclopedia of sustainability science and technology, Chap. 2. Springer, New York
Jossen A (2006) Fundamentals of battery dynamics. J Power Sources 154(2):530–538
Pritam, Arya A, Sharma AL (2019) Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J Mater Sci 54(9):7131–7155. https://doi.org/10.1007/s10853-019-03381-3
Pritam, Arya A, Sharma AL (2019) Selection of best composition of Na+ ion conducting PEO-PEI blend solid polymer electrolyte based on structural, electrical, and dielectric spectroscopic analysis. Ionics 26:745–766
Goodenough JB, Singh P (2015) Solid electrolytes in rechargeable electrochemical cells. J Electrochem Soc 162(14):A2387–A2392
Wu B, Wang S, Evans WJ, Deng DZ, Yang J, Xiao J (2016) Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J Mater Chem A 4(40):15266–15280
Bhattacharya M (2016) Polymer nanocomposites—a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 9(4):262
Xue Z, He D, Xie X (2015) Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3(38):19218–19253
Jiang Y, Yan X, Ma Z, Mei P, Xiao W, You Q, Zhang Y (2018) Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 10(11):1237
Saito Y, Morimura W, Kuse S, Kuratani R, Nishikawa S (2017) Influence of the morphological characteristics of separator membranes on ionic mobility in lithium secondary batteries. J Phys Chem C 121(5):2512–2520
Xia L, Yu L, Hu D, Chen GZ (2017) Electrolytes for electrochemical energy storage. Mater Chem Front 1(4):584–618
Cheng XB, Zhao CZ, Yao YX, Liu H, Zhang Q (2019) Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 5:74–96
Yue J, Ma J, Zhang J, Zhao J, Dong S, Liu Z, Cui G, Chen L (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater 5:139–164
Dirican M, Yan C, Zhu P, Zhang X (2019) Composite solid electrolytes for all-solid-state lithium batteries. Mater Sci Eng R 136:27–46
Meesala Y, Jena A, Chang H, Liu RS (2017) Recent advancements in Li-ion conductors for all-solid-state Li ion batteries. ACS Energy Lett 2:2734–2751
Schnell J, Günther T, Knoche T, Vieider C, Köhler L, Just A, Reinhart G (2018) All-solid-state lithium-ion and lithium metal batteries–paving the way to large-scale production. J Power Sources 382:160–175
Tong B, Wang J, Liu Z, Ma L, Zhou Z, Peng Z (2018) Identifying compatibility of lithium salts with LiFePO4 cathode using a symmetric cell. J Power Sources 384:80–85
Famprikis T, Canepa P, Dawson JA, Islam MS, Masquelier C (2019) Fundamentals of inorganic solid-state electrolytes for batteries. Nat Mater 18:1278–1291
Xia S, Wu X, Zhang Z, Cui Y, Liu W (2019) Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 5(4):753–785
Kerman K, Luntz A, Viswanathan V, Chiang YM, Chen Z (2017) Review—Practical challenges hindering the development of solid state Li ion batteries. J Electrochem Soc 164(7):A1731–A1744
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23(3):497–540
Arya A, Sharma AL (2019) Electrolyte for energy storage/conversion (Li+, Na+, Mg2+) devices based on PVC and their associated polymer: a comprehensive review. J Solid State Electrochem 23(4):997–1059
Arya A, Sharma AL (2020) Polymer Nanocomposites: synthesis and characterization. In: Wiesner M, Bottero JY (eds) Environmental nanotechnology, vol 4. Springer, Cham, pp 265–315
Xu K, Wang C (2016) Batteries: widening voltage windows. Nat Energy 1(10):16161
Kong L, Li C, Jiang J, Pecht MG (2018) Li-ion battery fire hazards and safety strategies. Energies 11(9):2191
Liu K, Liu Y, Lin D, Pei A, Cui Y (2018) Materials for lithium-ion battery safety. Sci Adv 4(6):eaas9820
Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Arya A, Sharma AL (2018) Structural, microstructural and electrochemical properties of dispersed-type polymer nanocomposite films. J Phys D Appl Phys 51(4):045504
Arya A, Sharma AL (2018) Structural, electrical properties and dielectric relaxations in Na+-ion-conducting solid polymer electrolyte. J Phys Condens Matter 30(16):165402
Petronico A, Moneypenny TP, Nicolau BG, Moore JS, Nuzzo RG, Gewirth AA (2018) Solid-liquid lithium electrolyte nanocomposites derived from porous molecular cages. J Am Chem Soc 24:7504–7509
Rosso M, Brissot C, Teyssot A, Dollé M, Sannier L, Tarascon JM, Bouchet R, Lascaud S (2006) Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim Acta 51(25):5334–5340
Wu B, Liu Q, Mu D, Xu H, Wang L, Shi L, Gai L, Wu F (2016) Suppression of lithium dendrite growth by introducing a low reduction potential complex cation in the electrolyte. RSC Adv 6(57):51738–51746
Bai P, Li J, Brushett FR, Bazant MZ (2016) Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ Sci 9(10):3221–3229
Ilott AJ, Mohammadi M, Chang HJ, Grey CP, Jerschow A (2016) Real-time 3D imaging of microstructure growth in battery cells using indirect MRI. Proc Natl Acad Sci USA 113(39):10779–10784
Mehdi BL, Stevens A, Qian J, Park C, Xu W, Henderson WA, Zhang JG, Mueller KT, Browning ND (2016) The impact of Li grain size on coulombic efficiency in Li batteries. Sci Rep 6:34267
Ma J, Chen B, Wang L, Cui G (2018) Progress and prospect on failure mechanisms of solid-state lithium batteries. J Power Sources 392:94–115
Brissot C, Rosso M, Chazalviel JN, Baudry P, Lascaud S (1998) In situ study of dendritic growth in lithium/PEO-salt/lithium cells. Electrochim Acta 43:1569–1574
Brissot C, Rosso M, Chazalviel JN (1999) Dendritic growth mechanisms in lithium/polymer cells. J Power Sources 81:925–929
Dollé M, Sannier L, Beaudoin B (2002) Live scanning electron microscope observations of dendritic growth in lithium/polymer cells. Electrochem Solid-state Lett 5:A286–A289
Rosso M, Brissot C, Teyssot A, Dollé M, Sannier L, Tarascon JM, Bouchet R, Lascaud S (2006) Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim Acta 51:5334–5340
Harry KJ, Hallinan DT, Parkinson DY, MacDowell AA, Balsara NP (2014) Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat Mater 13:69–73
Yang L, Wang Z, Feng Y, Tan R, Zuo Y, Gao R, Zhao Y, Han L, Wang Z, Pan F (2017) Flexible composite solid electrolyte facilitating highly stable “soft contacting” Li–electrolyte interface for solid state lithium-ion batteries. Adv Energy Mater 7:1701437
Wang C, Yang Y, Liu X, Zhong H, Xu H, Xu Z, Shao H, Ding F (2017) Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl Mater Interfaces 9:13694–13702
Wu H, Zhuo D, Kong D, Cui Y (2014) Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat Commun 5:5193
Li W, Yao H, Yan K, Zheng G, Liang Z, Chiang YM, Cui Y (2015) The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat Commun 6:7436
Zhao CZ, Zhang XQ, Cheng XB, Zhang R, Xu R, Chen PY, Peng HJ, Huang JQ, Zhang Q (2017) An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc Natl Acad Sci USA 114(42):11069–11074
Kim SH, Choi KH, Cho SJ, Kil EH, Lee SY (2013) Mechanically compliant and lithium dendrite growth-suppressing composite polymer electrolytes for flexible lithium-ion batteries. J Mater Chem A 1(16):4949–4955
Luo W, Zhou L, Fu K, Yang Z, Wan J, Manno M, Yao Y, Zhu H, Yang B, Hu L (2015) A thermally conductive separator for stable Li metal anodes. Nano Lett 15(9):6149–6154
Wu JY, Ling SG, Yang Q, Li H, Xu XX, Chen LQ (2016) Forming solid electrolyte interphase in situ in an ionic conducting Li1.5Al0.5Ge1.5(PO4) 3-polypropylene (PP) based separator for Li-ion batteries. Chin Phys B 25:78204
Zhao CZ, Chen PY, Zhang R, Chen X, Li BQ, Zhang XQ, Cheng XB, Zhang Q (2018) An ion redistributor for dendrite-free lithium metal anodes. Sci Adv 4(11):eaat3446
Hendricks C, Williard N, Mathew S, Pecht M (2015) A failure modes, mechanisms, and effects analysis (FMMEA) of lithium-ion batteries. J Power Sources 297:113–120
Niitani T, Shimada M, Kawamura K, Kanamura K (2005) Characteristics of new-type solid polymer electrolyte controlling nano-structure. J Power Sources 146(1–2):386–390
Khan IM, Fish D, Delaviz Y, Smid J (1989) ABA triblock comb copolymers with oligo (oxyethylene) side chains as matrix for ion transport. Macromol Chem Phys 190(5):1069–1078
Kamigaito M, Ando T, Sawamoto M (2001) Metal-catalyzed living radical polymerization. Chem Rev 101(12):3689–3746
Holmberg S, Holmlund P, Wilén CE, Kallio T, Sundholm G, Sundholm F (2002) Synthesis of proton-conducting membranes by the utilization of preirradiation grafting and atom transfer radical polymerization techniques. J Polym Sci Part A Polym Chem 40(4):591–600
Grewal MS, Tanaka M, Kawakami H (2019) Bifunctional poly (ethylene glycol) based crosslinked network polymers as electrolytes for all-solid-state lithium ion batteries. Polym Int 68(4):684–693
Porcarelli L, Gerbaldi C, Bella F, Nair JR (2016) Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci Rep 6:19892
Cui Y, Liang X, Chai J, Cui Z, Wang Q, He W, Feng J (2017) High performance solid polymer electrolytes for rechargeable batteries: a self-catalyzed strategy toward facile synthesis. Adv Sci 4(11):1700174
Huang S, Cui Z, Qiao L, Xu G, Zhang J, Tang K, Cui G (2019) An in situ polymerized solid polymer electrolyte enables excellent interfacial compatibility in lithium batteries. Electrochim Acta 299:820–827
Nair JR, Colò F, Kazzazi A, Moreno M, Bresser D, Lin R, Appetecchi GB (2019) Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J Power Sources 412:398–407
Nair JR, Destro M, Bella F, Appetecchi GB, Gerbaldi C (2016) Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J Power Sources 306:258–267
Nair JR, Porcarelli L, Bella F, Gerbaldi C (2015) Newly elaborated multipurpose polymer electrolyte encompassing RTILs for smart energy-efficient devices. ACS Appl Mater Interfaces 7:12961–12971
Falco M, Simari C, Ferrara C, Nair JR, Meligrana G, Bella F, Gerbaldi C (2019) Understanding the effect of UV-induced crosslinking on the physico-chemical properties of highly performing PEO/LiTFSI-based polymer electrolytes. Langmuir 35:8210–8219
Nair JR, Shaji I, Ehteshami N, Thum A, Diddens D, Heuer A, Winter M (2019) Solid polymer electrolytes for lithium metal battery via thermally induced cationic ring-opening polymerization (CROP) with an insight into the reaction mechanism. Chem Mater 31(9):3118–3133
Nuyken O, Pask S (2013) Ring-opening polymerization—an introductory review. Polymers 5(2):361–403
Ehteshami N, Eguia-Barrio A, de Meatza I, Porcher W, Paillard E (2018) Adiponitrile-based electrolytes for high voltage, graphite-based Li-ion battery. J Power Sources 397:52–58
Zaheer M, Xu H, Wang B, Li L, Deng Y (2020) An in situ polymerized comb-like PLA/PEG-based solid polymer electrolyte for lithium metal batteries. J Electrochem Soc 167(7):070504
Arya A, Sharma AL (2018) Effect of salt concentration on dielectric properties of Li-ion conducting blend polymer electrolytes. J Mater Sci Mater Electron 29(20):17903–17920
Arya A, Saykar Nilesh G, Sharma AL (2019) Impact of shape (nanofiller vs. nanorod) of TiO2 nanoparticle on free-standing solid polymeric separator for energy storage/conversion devices. J Appl Polym Sci 136(16):47361
Zhang X, Xie J, Shi F, Lin D, Liu Y, Liu W, Pei A, Gong Y, Wang H, Liu K, Xiang Y, Cui Y (2018) Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett 18(6):3829–3838
Liang X, Han D, Wang Y, Lan L, Mao J (2018) Preparation and performance study of a PVDF-LATP ceramic composite polymer electrolyte membrane for solid-state batteries. RSC Adv 8(71):40498–40504
Young WS, Kuan WF, Epps TH (2014) Block copolymer electrolytes for rechargeable lithium batteries. J Polym Sci Part B Polym Phys 52(1):1–16
Giacomelli C, Schmidt V, Aissou K, Borsali R (2010) Block copolymer systems: from single chain to self-assembled nanostructures. Langmuir 26(20):15734–15744
Young WS, Epps TH (2012) Ionic conductivities of block copolymer electrolytes with various conducting pathways: Sample preparation and processing considerations. Macromolecules 45(11):4689–4697
Cho BK, Jain A, Gruner SM, Wiesner U (2004) Mesophase structure-mechanical and ionic transport correlations in extended amphiphilic dendrons. Science 305(5690):1598–1601
Mindemark J, Lacey MJ, Bowden T, Brandell D (2018) Beyond PEO—alternative host materials for Li + -conducting solid polymer electrolytes. Prog Polym Sci 81:114–143
Zhang J, Yang J, Dong T, Zhang M, Chai J, Dong S, Cui G (2018) Aliphatic polycarbonate-based solid-state polymer electrolytes for advanced lithium batteries: advances and perspective. Small 14(36):1800821
Ren S, Chang H, He L, Dang X, Fang Y, Zhang L, Li H, Hu Y, Lin Y (2013) Preparation and ionic conductive properties of all-solid polymer electrolytes based on multiarm star block polymers. J Appl Polym Sci 129(3):1131–1142
Zhang J, Ma C, Liu J, Chen L, Pan A, Wei W (2016) Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J Membr Sci 509:138–148
Xu H, Wang A, Liu X, Feng D, Wang S, Chen J, Zhang L (2018) A new fluorine-containing star-branched polymer as electrolyte for all-solid-state lithium-ion batteries. Polymer 146:249–255
Li Y, Zhang W, Dou Q, Wong KW, Ng KM (2019) Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J Mater Chem A 7(7):3391–3398
Zhang X, Ji L, Toprakci O, Liang Y, Alcoutlabi M (2011) Electrospun nanofiber-based anodes, cathodes, and separators for advanced lithium-ion batteries. Polym Rev 51(3):239–264
Liu W, Liu N, Sun J, Hsu PC, Li Y, Lee HW, Cui Y (2015) Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett 15(4):2740–2745
Zhu P, Yan C, Dirican M, Zhu J, Zang J, Selvan RK, Chung CC, Jia H, Li Y, Kiyak Y, Wu N, Zhang X (2018) Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J Mater Chem A 6(10):4279–4285
Lu Q, He YB, Yu Q, Li B, Kaneti YV, Yao Y, Kang F, Yang QH (2017) Dendrite-free, high-rate, long-life lithium metal batteries with a 3D cross-linked network polymer electrolyte. Adv Mater 29(13):1604460
Kim GT, Appetecchi GB, Carewska M, Joost M, Balducci A, Winter M, Passerini S (2010) UV cross-linked, lithium-conducting ternary polymer electrolytes containing ionic liquids. J Power Sources 195(18):6130–6137
Lin YC, Ito K, Yokoyama H (2018) Solid polymer electrolyte based on crosslinked polyrotaxane. Polymer 136:121–127
Ji J, Li B, Zhong WH (2011) An ultraelastic poly (ethylene oxide)/soy protein film with fully amorphous structure. Macromolecules 45(1):602–606
Younesi R, Veith GM, Johansson P, Edström K, Vegge T (2015) Lithium salts for advanced lithium batteries: Li–metal, Li–O2 and Li–S. Energy Environ Sci. https://doi.org/10.1039/c5ee01215e.8(7),1905-1922
Ahmad S (2009) RETRACTED ARTICLE: polymer electrolytes: characteristics and peculiarities. Ionics 15(3):309–321
Ue M (1994) Mobility and ionic association of lithium and quaternary ammonium salts in propylene carbonate and γ-butyrolactone. J Electrochem Soc 141(12):3336–3342
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4418
Henderson WA (2014) Electrolytes for lithium and lithium-ion batteries, vol 58. Springer, New York
Niedzicki L, Grugeon S, Laruelle S, Judeinstein P, Bukowska M, Prejzner J, Szczeciñski P, Wieczorek W, Armand M (2011) New covalent salts of the 4 + v class for Li batteries. J Power Sources 196(20):8696–8700
Kim HS, Jeong CS (2011) Electrochemical properties of binary electrolytes for lithium-sulfur batteries. Bull Korean Chem Soc 32(10):3682–3686
Read J (2006) Ether-based electrolytes for the lithium/oxygen organic electrolyte battery. J Electrochem Soc 153(1):A96–A100
Gutmann V (1976) Solvent effects on the reactivities of organometallic compounds. Coord Chem Rev 18(2):225–255
Linert W, Camard A, Armand M, Michot C (2002) Anions of low Lewis basicity for ionic solid state electrolytes. Coord Chem Rev 226(1–2):137–141
Schmeisser M, Illner P, Puchta R, Zahl A, Van Eldik R (2012) Gutmann donor and acceptor numbers for ionic liquids. Chem Eur J 18(35):10969–10982
Wei W, Xu Z, Xu L, Zhang X, Xiong H, Yang J (2018) Flexible ionic conducting elastomers for all-solid-state room-temperature lithium batteries. ACS Appl Energy Mater 1(12):6769–6773
Garcia-Calvo O, Lago N, Devaraj S, Armand M (2016) Cross-linked solid polymer electrolyte for all-solid-state rechargeable lithium batteries. Electrochim Acta 220:587–594
Rupp B, Schmuck M, Balducci A, Winter M, Kern W (2008) Polymer electrolyte for lithium batteries based on photochemically crosslinked poly(ethylene oxide) and ionic liquid. Eur Polymer J 44(9):2986–2990
Souzandeh H, Johnson KS, Wang Y, Bhamidipaty K, Zhong WH (2016) Soy-protein-based nanofabrics for highly efficient and multifunctional air filtration. ACS Appl Mater Interfaces 8(31):20023–20031
Fu X, Jewel Y, Wang Y, Liu J, Zhong WH (2016) Decoupled ion transport in a protein-based solid ion conductor. J Phys Chem Lett 7(21):4304–4310
Yuan B, Luo G, Liang J, Cheng F, Zhang W, Chen J (2019) Self-assembly synthesis of solid polymer electrolyte with carbonate terminated poly(ethylene glycol) matrix and its application for solid state lithium battery. J Energy Chem 38:55–59
Xiao Z, Zhou B, Wang J, Zuo C, He D, Xie X, Xue Z (2019) PEO-based electrolytes blended with star polymers with precisely imprinted polymeric pseudo-crown ether cavities for alkali metal ion batteries. J Membr Sci 576:182–189
Murray V, Hall DS, Dahn JR (2019) A guide to full coin cell making for academic researchers. J Electrochem Soc 166(2):A329–A333
Lagadec MF, Zahn R, Wood V (2019) Characterization and performance evaluation of lithium-ion battery separators. Nat Energy 4:16–25
Doyle M, Fuller TF, Newman J (1994) The importance of the lithium ion transference number in lithium/polymer cells. Electrochim Acta 39(13):2073–2081
Zhou W, Wang S, Li Y, Xin S, Manthiram A, Goodenough JB (2016) Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J Am Chem Soc 138(30):9385–9388
Li H, Li M, Siyal SH, Zhu M, Le Lan J, Sui G, Yu Y, Zhong W, Yang X (2018) A sandwich structure polymer/polymer-ceramics/polymer gel electrolytes for the safe, stable cycling of lithium metal batteries. J Membr Sci 555:169–176
Lian F, Guan HY, Wen Y, Pan XR (2014) Polyvinyl formal based single-ion conductor membranes as polymer electrolytes for lithium ion batteries. J Membr Sci 469:67–72
Bae J, Li Y, Zhang J, Zhou X, Zhao F, Shi Y, Goodenough JB, Yu G (2018) A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angewandte Chemie Int Ed 57(8):2096–2100
Leo CJ, Rao GVS, Chowdari BVR (2002) Studies on plasticized PEO–lithium triflate–ceramic filler composite electrolyte system. Solid State Ion 148(1–2):159–171
Liu W, Liu N, Sun J, Hsu P-C, Li Y, Lee H-W, Cui Y (2015) Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett 15:2740–2745
Wang Y-J, Pan Y, Chen L (2005) Ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with Li1.3Al0.3Ti1.7(PO4)3 salt. Mater Chem Phys 92:354–360
Chen S, Wang J, Zhang Z, Wu L, Yao L, Wei Z, Xu X (2018) In-situ preparation of poly (ethylene oxide)/Li3PS4 hybrid polymer electrolyte with good nanofiller distribution for rechargeable solid-state lithium batteries. J Power Sources 387:72–80
Homma K, Yonemura M, Kobayashi T, Nagao M, Hirayama M, Kanno R (2011) Crystal structure and phase transitions of the lithium ionic conductor Li3PS4. Solid State Ion 182(1):53–58
Chen L, Li Y, Li SP, Fan LZ, Nan CW, Goodenough JB (2018) PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 46:176–184
Liu L, Chu L, Jiang B, Li M (2019) Li1.4Al0.4Ti1.6(PO4)3 nanoparticle-reinforced solid polymer electrolytes for all-solid-state lithium batteries. Solid State Ion 331:89–95
Aldalur I, Martinez-Ibañez M, Piszcz M, Rodriguez-Martinez LM, Zhang H, Armand M (2018) Lowering the operational temperature of all-solid-state lithium polymer cell with highly conductive and interfacially robust solid polymer electrolytes. J Power Sources 383:144–149
Aldalur I, Martinez-Ibañez M, Piszcz M, Zhang H, Armand M (2018) Self-standing highly conductive solid electrolytes based on block copolymers for rechargeable all-solid-state lithium-metal batteries. Batter Supercaps 1(4):149–159
Duan H, Yin YX, Zeng XX, Li JY, Shi JL, Shi Y, Wen R, Guo YG, Wan LJ (2018) In-situ plasticized polymer electrolyte with double-network for flexible solid-state lithium-metal batteries. Energy Storage Mater 10:85–91
Nguyen HD, Kim GT, Shi J, Paillard E, Judeinstein P, Lyonnard S, Bresser D, Iojoiu C (2018) Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries. Energy Environ Sci 11(11):3298–3309
Xiao Y, Jiang L, Liu Z, Yuan Y, Yan P, Zhou C, Lei J (2017) Effect of phase separation on the crystallization of soft segments of green waterborne polyurethanes. Polym Test. https://doi.org/10.1016/j.polymertesting.2017.03.029.60,160-165
Xu C, Huang Y, Tang L, Hong Y (2017) Low-initial-modulus biodegradable polyurethane elastomers for soft tissue regeneration. ACS Appl Mater Interfaces 9(3):2169–2180
Karimi MB, Khanbabaei G, Sadeghi GMM (2017) Vegetable oil-based polyurethane membrane for gas separation. J Membr Sci 527:198–206
Bao J, Shi G, Tao C, Wang C, Zhu C, Cheng L, Qian G, Chen C (2018) Polycarbonate-based polyurethane as a polymer electrolyte matrix for all-solid-state lithium batteries. J Power Sources 389:84–92
Lee YH, Kim JS, Noh J, Lee I, Kim HJ, Choi S, Seo J, Jeon S, Kim TS, Lee JY, Choi JW (2013) Wearable textile battery rechargeable by solar energy. Nano Lett 13(11):5753–5761
Wang S, Jeung S, Min K (2010) The effects of anion structure of lithium salts on the properties of in situ polymerized thermoplastic polyurethane electrolytes. Polymer 51(13):2864–2871
Cong B, Song Y, Ren N, Xie G, Tao C, Huang Y, Xu G, Bao J (2018) Polyethylene glycol-based waterborne polyurethane as solid polymer electrolyte for all-solid-state lithium ion batteries. Mater Des 142:221–228
Choudhary S, Sengwa RJ (2017) Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim Acta 247:924–941
Zhang J, Zang X, Wen H, Dong T, Chai J, Li Y, Yue L (2017) High-voltage and free-standing poly (propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery. J Mater Chem A 5(10):4940–4948
Gopalan AI, Santhosh P, Manesh KM, Nho JH, Kim SH, Hwang CG, Lee KP (2008) Development of electrospun PVdF-PAN membrane-based polymer electrolytes for lithium batteries. J Membr Sci 325(2):683–690
Song MK, Kim YT, Cho JY, Cho BW, Popov BN, Rhee HW (2004) Composite polymer electrolytes reinforced by non-woven fabrics. J Power Sources 125(1):10–16
Jeong HS, Choi ESH, Lee SY, Kim JH (2012) Evaporation-induced, close-packed silica nanoparticle-embedded nonwoven composite separator membranes for high-voltage/high-rate lithium-ion batteries: advantageous effect of highly percolated, electrolyte-philic microporous architecture. J Membr Sci 415:513–519
Zhu Y, Wang F, Liu L, Xiao S, Chang Z, Wu Y (2013) Composite of a nonwoven fabric with poly(vinylidene fluoride) as a gel membrane of high safety for lithium ion battery. Energy Environ Sci. https://doi.org/10.1039/c2ee23564a.6(2),618-624
Pitawala J, Navarra MA, Scrosati B, Jacobsson P, Matic A (2014) Structure and properties of Li-ion conducting polymer gel electrolytes based on ionic liquids of the pyrrolidinium cation and the bis(trifluoromethanesulfonyl)imide anion. J Power Sources 245:830–835
Kuo PL, Tsao CH, Hsu CH, Chen ST, Hsu HM (2016) A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J Membr Sci 499:462–469
Lu Q, Fang J, Yang J, Yan G, Liu S, Wang J (2013) A novel solid composite polymer electrolyte based on poly(ethylene oxide) segmented polysulfone copolymers for rechargeable lithium batteries. J Membr Sci 425:105–112
Shi J, Yang Y, Shao H (2018) Co-polymerization and blending based PEO/PMMA/P(VDF-HFP) gel polymer electrolyte for rechargeable lithium metal batteries. J Membr Sci 547:1–10
Smith SA, Williams BP, Joo YL (2017) Effect of polymer and ceramic morphology on the material and electrochemical properties of electrospun PAN/polymer derived ceramic composite nanofiber membranes for lithium ion battery separators. J Membr Sci 526:315–322
Tsao CH, Kuo PL (2015) Poly(dimethylsiloxane) hybrid gel polymer electrolytes of a porous structure for lithium ion battery. J Membr Sci 489:36–42
Wang X, Zhang Y, Zhang X, Liu T, Lin YH, Nan CW (2018) Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl Mater Interfaces 10(29):24791–24798
Zekoll S, Marriner-Edwards C, Hekselman AKO, Kasemchainan J, Kuss C, Armstrong DEJ, Cai D, Wallace RJ, Richter FH, Thijssen JHJ, Bruce PG (2018) Hybrid electrolytes with 3D bicontinuous ordered ceramic and polymer microchannels for all-solid-state batteries. Energy Environ Sci 11(1):185–201
Wang B, Rivard E, Manners I (2002) A new high-yield synthesis of Cl3P = NSiMe3, a monomeric precursor for the controlled preparation of high molecular weight polyphosphazenes. Inorg Chem 41(7):1690–1691
Yu S, Schmohl S, Liu Z, Hoffmeyer M, Schön N, Hausen F, Tempel H, Kungl H, Wiemhöfer HD, Eichel RA (2019) Insights into a layered hybrid solid electrolyte and its application in long lifespan high-voltage all-solid-state lithium batteries. J Mater Chem A 7(8):3882–3894
Kwon SJ, Kim DG, Shim J, Lee JH, Baik JH, Lee JC (2014) Preparation of organic/inorganic hybrid semi-interpenetrating network polymer electrolytes based on poly (ethylene oxide-co-ethylene carbonate) for all-solid-state lithium batteries at elevated temperatures. Polymer 55(12):2799–2808
Shin WK, Cho J, Kannan AG, Lee YS, Kim DW (2016) Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci Rep 6:26332
Zhang J, Li X, Li Y, Wang H, Ma C, Wang Y, Hu S, Wei W (2018) Cross-linked nanohybrid polymer electrolytes with POSS cross-linker for solid-state lithium ion batteries. Front Chem 6:186
Zhang Y, Lu W, Cong L, Liu J, Sun L, Mauger A, Liu J (2019) Cross-linking network based on Poly (ethylene oxide): solid polymer electrolyte for room temperature lithium battery. J Power Sources 420:63–72
Zhu M, Tan C, Fang Q, Gao L, Sui G, Yang X (2016) High performance and biodegradable skeleton material based on soy protein isolate for gel polymer electrolyte. ACS Sustai Chem Eng 4(9):4498–4505
Fu X, Li C, Wang Y, Kovatch LP, Scudiero L, Liu J, Zhong W (2018) Building ion-conduction highways in polymeric electrolytes by manipulating protein configuration. ACS Appl Mater Interfaces 10(5):4726–4736
Fu X, Wang Y, Fan X, Scudiero L, Zhong WH (2018) Core-shell hybrid nanowires with protein enabling fast ion conduction for high-performance composite polymer electrolytes. Small 14(49):1803564
Kumar B, Scanlon LG (1994) Polymer-ceramic composite electrolytes. J Power Sources 52(2):261–268
Wang X, Fu X, Wang Y, Zhong W (2016) A protein-reinforced adhesive composite electrolyte. Polymer 106:43–52
Chen F, Yang D, Zha W, Zhu B, Zhang Y, Li J, Gu Y, Shen Q, Zhang L, Sadoway DR (2017) Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries. Electrochim Acta 258:1106–1114
Bae J, Li Y, Zhao F, Zhou X, Ding Y, Yu G (2018) Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater 15:46–52
Sun Y, Zhan X, Hu J, Wang Y, Gao S, Shen Y, Cheng YT (2019) Improving ionic conductivity with bimodal-sized Li7La3Zr2O12 fillers for composite polymer electrolytes. ACS Appl Mater Interfaces 11:13
Zhu L, Zhu P, Yao S, Shen X, Tu F (2019) High-performance solid PEO/PPC/LLTO-nanowires polymer composite electrolyte for solid-state lithium battery. Int J Energy Res 43:4854–4866
Sun Z, Li Y, Zhang S, Shi L, Wu H, Bu H, Ding S (2019) G-C3N4 nanosheets enhanced solid polymer electrolytes with excellent electrochemical performance, mechanical properties, and thermal stability. J Mater Chem A 7(18):11069–11076
Chen L, Fan LZ (2018) Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte. Energy Storage Mater 15:37–45
Mindemark J, Sun B, Törmä E, Brandell D (2015) High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature. J Power Sources 298:166–170
Sun B, Mindemark J, Edström K, Brandell D (2014) Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ion 262:738–742
Sun B, Mindemark J, Edström K, Brandell D (2015) Realization of high performance polycarbonate-based Li polymer batteries. Electrochem Commun 52:71–74
Mindemark J, Imholt L, Montero J, Brandell D (2016) Allyl ethers as combined plasticizing and crosslinkable side groups in polycarbonate-based polymer electrolytes for solid-state Li batteries. J Polym Sci Part A Polym Chem 54(14):2128–2135
Kimura K, Yajima M, Tominaga Y (2016) A highly-concentrated poly (ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem Commun 66:46–48
Wang A, Xu H, Zhou Q, Liu X, Li Z, Gao R, Wu N, Guo Y, Li H, Zhang L (2016) A new all-solid-state hyperbranched star polymer electrolyte for lithium ion batteries: synthesis and electrochemical properties. Electrochim Acta 212:372–379
Wang S, Wang A, Yang C, Gao R, Liu X, Chen J, Wang Z, Zeng Q, Liu X, Zhou H, Zhang L (2018) Six-arm star polymer based on discotic liquid crystal as high performance all-solid-state polymer electrolyte for lithium-ion batteries. J Power Sources 395:137–147
Chaudoy V, Ghamouss F, Luais E, Tran-Van F (2016) Cross-linked polymer electrolytes for Li-based batteries: from solid to gel electrolytes. Ind Eng Chem Res 55(37):9925–9933
Sakakibara T, Kitamura M, Honma T, Kohno H, Uno T, Kubo M, Itoh T (2019) Cross-linked polymer electrolyte and its application to lithium polymer battery. Electrochimica Acta 296:1018–1026
Pan Q, Zheng Y, Kota S, Huang W, Wang S, Qi H, Li CY (2019) 2D MXene-containing polymer electrolytes for all-solid-state lithium metal batteries. Nanoscale Adv 1(1):395–402
Wang B, Tang M, Wu Y, Chen Y, Jiang C, Zhuo S, Zhu S, Wang C (2019) A 2D layered natural ore as a novel solid-state electrolyte. ACS Appl Energy Mater 2(8):5909–5916
Tang W, Tang S, Zhang C, Ma Q, Xiang Q, Yang YW, Luo J (2018) Simultaneously enhancing the thermal stability, mechanical modulus, and electrochemical performance of solid polymer electrolytes by incorporating 2D sheets. Adv Energy Mater 8(24):1800866
Tang W, Tang S, Guan X, Zhang X, Xiang Q, Luo J (2019) High-performance solid polymer electrolytes filled with vertically aligned 2D materials. Adv Funct Mater 29(16):1900648
Piana G, Bella F, Geobaldo F, Meligrana G, Gerbaldi C (2019) PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J Energy Storage 26:100947
Fu J, Lu Q, Shang D, Chen L, Jiang Y, Xu Y, Yuan S (2018) A novel room temperature POSS ionic liquid-based solid polymer electrolyte. J Mater Sci 53(11):8420–8435
Cui W, Tang DY, Gong ZL, Guo YD (2012) Performance enhancement induced by electrospinning of polymer electrolytes based on poly(methyl methacrylate-co-2-acrylamido-2-methylpropanesulfonic acid lithium). J Mater Sci 47:6276–6285. https://doi.org/10.1007/s10853-012-6547-3
Zhang N, He J, Han W, Wang Y (2019) Composite solid electrolyte PEO/SN/LiAlO2 for a solid-state lithium battery. J Mater Sci 54(13):9603–9612. https://doi.org/10.1007/s10853-019-03535-3
Guo HL, Sun H, Jiang ZL, Luo CS, Gao MY, Wei MH, Zhou HJ (2019) A new type of composite electrolyte with high performance for room-temperature solid-state lithium battery. J Mater Sci 54(6):4874–4883. https://doi.org/10.1007/s10853-018-03188-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arya, A., Sharma, A.L. A glimpse on all-solid-state Li-ion battery (ASSLIB) performance based on novel solid polymer electrolytes: a topical review. J Mater Sci 55, 6242–6304 (2020). https://doi.org/10.1007/s10853-020-04434-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04434-8