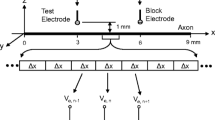
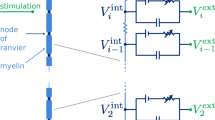
Abstract
The purpose of this modeling study is to develop a novel method to block nerve conduction by high frequency biphasic stimulation (HFBS) without generating initial action potentials. An axonal conduction model including both ion concentrations and membrane ion pumps is used to analyze the axonal response to 1 kHz HFBS. The intensity of HFBS is increased in multiple steps while maintaining the intensity at a sub-threshold level to avoid generating an action potential. Axonal conduction block by HFBS is defined as the failure of action potential propagation at the site of HFBS. The simulation analysis shows that step-increases in sub-threshold intensity during HFBS can successfully block axonal conduction without generating an initial response because the excitation threshold of the axon can be gradually increased by the sub-threshold HFBS. The mechanisms underlying the increase in excitation threshold involve changes in intracellular and extracellular sodium and potassium concentration, change in the resting potential, partial inactivation of the sodium channel and partial activation of the potassium channel by HFBS. When the excitation threshold reaches a sufficient level, an acute block occurs first and after additional sub-threshold HFBS it is followed by a post-stimulation block. This study indicates that step-increases in sub-threshold HFBS intensity induces a gradual increase in axonal excitation threshold that may allow HFBS to block nerve conduction without generating an initial response. If this finding is proven to be true in human, it will significantly impact clinical applications of HFBS to treat chronic pain.







Similar content being viewed by others
Data availability
All data and materials are presented in this paper.
Code availability
Code is availability at the corresponding author’s lab and will be made available in public databases as suggested by the journal editor.
References
Apovian, C. M., Shah, S. N., Wolfe, B. M., Ikramuddin, S., Miller, C. J., Tweden, K. S., Billington, C. J., & Shikora, S. A. (2017). Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obesity Surgery, 27, 169–176.
Bowman, B. R., & McNeal, D. R. (1986). Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Applied Neurophysiology, 49, 121–138.
Boyce, W. E., & Diprima, R. C. (1997). Elementary differential equations and boundary value problems (6th ed., pp. 436–457). Wiley.
Franke, M., Vrabec, T., Wainright, J., Bhadra, N., Bhadra, N., & Kilgore, K. (2014). Combined KHFAC + DC nerve block without onset or reduced nerve conductivity after block. Journal of Neural Engineering, 11, 056012.
Frankenhaeuser, B., & Hodgkin, A. L. (1956). The after-effects of impulses in the giant nerve fibres of Loligo. Journal of Physiology, 131, 341–376.
Franz, D. N., & Perry, R. S. (1974). Mechanisms for differential block among single myelinated and non-myelinated axons by procaine. Journal of Physiology, 236, 193–210.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Miles, J. D., Kilgore, K. L., Bhadra, N., & Lahowetz, E. A. (2007). Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. Journal of Neural Engineering, 4, 390–398.
Mullins, L. J., & Brinley, F. J., Jr. (1969). Potassium fluxes in dialyzed squid axons. Journal of General Physiology, 53, 704–740.
Mullins, L. J., & Noda, K. (1963). The Influence of sodium-free solutions on the membrane potential of frog muscle fibers. Journal of General Physiology, 47, 117–132.
Rang, H. P., & Ritchie, J. M. (1968). The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. Journal of Physiology, 196, 223–236.
Rattay, F. (1989). Analysis of models for extracellular fiber stimulation. IEEE Transactions on Biomedical Engineering, 36, 676–682.
Rattay, F., & Aberham, M. (1993). Modeling axon membranes for functional electrical stimulation. IEEE Transactions on Biomedical Engineering, 40, 1201–1209.
Scriven, D. R. (1981). Modeling repetitive firing and bursting in a small unmyelinated nerve fiber. Biophysical Journal, 35, 715–730.
Shapiro, K., Guo, W., Armann, K., Pace, N., Shen, B., Wang, J., Beckel, J., de Groat, W., & Tai, C. (2021). Pudendal nerve block by low-frequency (≤1 kHz) biphasic electrical stimulation. Neuromodulation, 24, 1012–1027.
Soin, A., Shah, N. S., & Fang, Z. P. (2015). High-frequency electrical nerve block for postamputation pain: A pilot study. Neuromodulation, 18, 197–205.
Tai, C., de Groat, W. C., & Roppolo, J. R. (2005). Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Transactions on Biomedical Engineering, 52, 1323–1332.
Tai, C., Wang, J., Chancellor, M. B., Roppolo, J. R., & de Groat, W. C. (2008). Influence of temperature on pudendal nerve block induced by high frequency biphasic electrical current. Journal of Urology, 180, 1173–1178.
Thomas, R. C. (1972). Electrogenic sodium pump in nerve and muscle cells. Physiological Reviews, 52, 563–594.
Wang, Z., Pace, N., Cai, H., Shen, B., Wang, J., Roppolo, J. R., de Groat, W. C., & Tai, C. (2020). Poststimulation block of pudendal nerve conduction by high-frequency (kHz) biphasic stimulation in cats. Neuromodulation, 23, 747–753.
Whitwam, J. G., & Kidd, C. (1975). The use of direct current to cause selective block of large fibers in peripheral nerves. British Journal of Anaesthesia, 47, 1123–1133.
Yang, G., Xiao, Z., Wang, J., et al. (2017). Post-stimulation block of frog sciatic nerve by high-frequency (kHz) biphasic stimulation. Medical & Biological Engineering & Computing, 55, 585–593.
Yi, G., & Grill, W. M. (2020). Kilohertz waveforms optimized to produce closed-state Na+ channel inactivation eliminate onset response in nerve conduction block. PLOS Computational Biology, 16(6), e1007766.
Zhang, X., Roppolo, J. R., de Groat, W. C., & Tai, C. (2006). Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Transactions on Biomedical Engineering, 53, 2445–2454.
Zhong, Y., Wang, J., Beckel, J., de Groat, W., & Tai, C. (2021a). Model analysis of post-stimulation effect on axonal conduction and block. IEEE Transactions on Biomedical Engineering, 68, 2974–2985.
Zhong, Y., Wang, J., Beckel, J., de Groat, WC., & Tai, C. (2021b). Mechanisms underlying poststimulation block induced by high-frequency biphasic stimulation. Neuromodulation. https://doi.org/10.1111/ner.13501. Epub ahead of print.
Funding
This study is funded by the National Institute of Neurological Disorders and Stroke under grant R01NS109198.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Tai is an inventor of a patent application related to this study. The other authors have no conflicts of interest.
Additional information
Communicated by Action Editor: Geir Halnes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The total ionic current \({I}_{i,j}\) at the jth segment is described as follows:
\({I}_{Na}\) is the sodium (Na+) current; \({I}_{K}\) is the potassium (K+) current; \({I}_{L}\) is the leak current; \({I}_{Na-p}\) is the Na+ pump current. \({I}_{K-p}\) is the K+ pump current.
The Na+ current at jth segment is described as:
where \({G}_{Na}\) is the maximal Na+ conductance (120 \({{k\Omega }^{-1}cm}^{-2}\)); m is the activation of Na+ channel; h is the inactivation of Na+ channel;\({V}_{n}\) is the change of the membrane potential relative to the resting membrane potential \({E}_{rest}\); \({V}_{Na}\) is the reduced equilibrium membrane potential of Na+ ions after subtracting the resting membrane potential \({E}_{rest}\); \({E}_{Na}\) is the Na+ equilibrium potential; \(R\) is the gas constant (8.3 \(J/(mol*K)\)); T is the temperature in Kelvin; F is the Faraday constant (96,485 \(C/mol\)). \({Na}_{in}\) is the Na+ concentration inside the axon with an initial value of 50 \(mmol/L\); \({Na}_{ps}\) is the Na+ concentration outside the axon in the periaxonal space with an initial value of \(440 mmol/L\) that equals to the constant Na+ concentration in the large extracellular space (\({Na}_{o}\)). The periaxonal space consists of a very thin layer of fluid (thickness of \(\mathrm{} 1.45\times {10}^{-2} \mu m\)) that separates the axonal membrane from the large extracellular space (Frankenhaeuser & Hodgkin, 1952; Scriven, 1981; Whitwam & Kidd, 1975). Ions can diffuse between the periaxonal space and the large extracellular space where the ion concentrations are maintained constant.
The K+ current at jth segment is described as:
where \({G}_{K}\) is the maximal K+ conductance (36 \({{k\Omega }^{-1}cm}^{-2}\)); n is the activation of K+ channel; \({V}_{K}\) is reduced equilibrium membrane potential of K+ ions after subtracting the resting membrane potential \({E}_{rest}\); \({E}_{K}\) is the K+ equilibrium potential; \({K}_{in}\) is the K+ concentration inside the axon with an initial value of 400 \(mmol/L\); \({K}_{ps}\) is the K+ concentration outside the axon in the periaxonal space with an initial value of \(20 mmol/L\) that equals the constant K+ concentration in the large extracellular space (\({K}_{o}\)).
At resting membrane potential \(({V}_{j}=0 mV)\), the total ionic current across the membrane \(({I}_{i,j})\) should be zero. Since at resting membrane potential \({I}_{Kp}={-I}_{K}, {I}_{Nap}={-I}_{Na}\), the leakage current at jth segment is described as follows:
where \({G}_{L}\) is the maximal conductance (0.3 \({{k\Omega }^{-1}cm}^{-2}\)) of the leakage current.
The resting membrane potential \({E}_{rest}\) is described by modified Goldman equation as follows (Mullins & Noda, 1963; Scriven, 1981):
where r is the Na–K pump ratio (Mullins & Brinley, 1969) and e is a constant (0.0566) representing the ratio of Na+ and K+ permeability.
where \({c}_{r}\) and \({d}_{r}\) are constants; In the squid axon \({c}_{r}\) has a value of 0.05 (Mullins & Brinley, 1969) and \({d}_{r}\) has a value of -1 since at initial resting state \(r=1.5\) (Thomas, 1972) and \(N{a}_{in}=50\).
The currents of Na+ and K+ pumps are described as (Mullins & Brinley, 1969; Scriven, 1981):
where \(a\) is a constant (0.0954). \({b}_{1}\) is the dissociation constant for \({K}_{ps}\), and \({b}_{2}\) is the dissociation constant for \({Na}_{in}\). The constants \({b}_{1}\) and \({b}_{2}\) are estimated to be 1 \(mmol/L\) and 30 \(mmol/L\), respectively (Mullins & Brinley, 1969; Rang & Ritchie, 1968).
The evolution equations for \(m, h, n\) are described as following (Hodgkin & Huxley, 1952; Scriven, 1981):
where \(ms, hs, ns\) are constants that equal 7.8, 3.1, 18.5, respectively. The initial values for m, h, n are 0.0204, 0.6988, and 0.1, respectively. \(\Phi\) is the coefficient of temperature.
The evolution equations for \({Na}_{in}, {K}_{in}, {Na}_{ps}, {K}_{ps}\) are described as follows (Frankenhaeuser & Hodgkin, 1956; Scriven, 1981):
where DNa \(and\) DK are diffusion constants for Na+ (0.1 \(\mu m/s\)) and K+ (0.1 \(\mu m/s\)) to diffuse between the periaxonal space and the large extracellular space, respectively. \(\uptheta\) (\(1.45\times {10}^{-2} \mu m\)) is the thickness of the periaxonal space (Frankenhaeuser & Hodgkin, 1956; Scriven, 1981).
Rights and permissions
About this article
Cite this article
Zhong, Y., Wang, J., Beckel, J. et al. High-frequency stimulation induces axonal conduction block without generating initial action potentials. J Comput Neurosci 50, 203–215 (2022). https://doi.org/10.1007/s10827-021-00806-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-021-00806-4