Abstract
Purpose
To evaluate whether mtDNA content at the blastocyst stage differs between embryos derived from fresh or vitrified sibling oocytes.
Material and methods
A retrospective analysis was performed between March 2017 and September 2018, including 504 blastocysts from 94 couples undergoing preimplantation genetic testing for aneuploidies (PGT-A), using fresh oocytes together with previously vitrified oocytes. Trophectoderm biopsies were performed and subjected to next generation sequencing.
Results
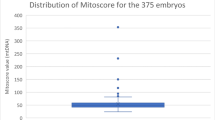
On average, 1.8 ± 1.0 oocyte vitrification cycles were performed per patient. Between fresh and vitrified cycles, no difference was observed between the number of fertilized oocytes (5.3 ± 4.2 versus 5.5 ± 3.0). Blastulation rate on day 5 per fertilized oocyte was significantly higher in the fresh group (62% ± 29% versus 44% ± 31%; p < 0.001). For the 504 biopsied blastocysts, 294 fresh versus 210 vitrified, no significant differences were found in the euploid rate, 40.5% versus 38.6% (p = 0.667), and mtDNA content, 30.1 (± 10.6) versus 30.0 (± 12.5) (p = 0.871), respectively. Regardless of the origin of the oocytes, aneuploid blastocysts contained significantly higher mtDNA values compared with the euploid ones (31.4 versus 28.0; p = 0.001). Furthermore, top-quality blastocysts had a significantly lower mtDNA content compared with moderate and poor-quality blastocysts (p < 0.001) and blastocysts biopsied on day 5 showed significantly lower mtDNA content compared with day 6 or day 7 blastocysts (p < 0.001). However, when analyzing the blastocyst mtDNA content according to the ploidy state, no differences were found for blastocyst quality or day of biopsy between blastocysts originating from fresh or vitrified oocytes.
Conclusion
Oocyte vitrification does not affect the mtDNA content of trophectoderm biopsies.





Similar content being viewed by others
References
Chamayou S, Sicali M, Alecci C, Ragolia C, Liprino A, Nibali D, et al. The accumulation of vitrified oocytes is a strategy to increase the number of euploid available blastocysts for transfer after preimplantation genetic testing. J Assist Reprod Genet. 2017;34(4):479–86.
Practice Committees of American Society for Reproductive Medicine: Society for Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012;98(3):644–9.
Fragouli E, Wells D. Mitochondrial DNA assessment to determine oocyte and embryo viability. Semin Reprod Med. 2015;33(06):401–9.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813.
Reynier P, May-Panloup P, Chretien M-F, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–9.
Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Current Opinion in Obstetrics and Gynecology. 2015;27(3):175–81.
Santos TA, El Shourbagy S. St. John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584–91.
Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20(3):346–53.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95.
May-Panloup P, Chretien M, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. In: Current Topics in Developmental Biology [Internet]. Elsevier; 4Y [cited 2019 Sep 3]. p. 51–83. Available from: https://linkinghub.elsevier.com/retrieve/pii/S007021530677003X
Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–87.
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–18.
Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–66.
Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15(12):2621–33.
Lei T, Guo N, Tan M, Li Y. Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J Huazhong Univ Sci Technol [Med Sci]. 2014;34(1):99–102.
Zhao J, Liu T, Jin S-B, Tomilin N, Castro J, Shupliakov O, et al. The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation. J Cell Sci. 2009;122(13):2252–62.
Zhao X-M, Du W-H, Wang D, Hao H-S, Liu Y, Qin T, et al. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol Reprod Dev. 2011;78(12):942–50.
Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26(9):2452–60.
Chen C, Han S, Liu W, Wang Y, Huang G. Effect of vitrification on mitochondrial membrane potential in human metaphase II oocytes. J Assist Reprod Genet. 2012;29(10):1045–50.
Manipalviratn S, Tong Z-B, Stegmann B, Widra E, Carter J, DeCherney A. Effect of vitrification and thawing on human oocyte ATP concentration. Fertil Steril. 2011 Apr;95(5):1839–41.
Nazmara Z, Salehnia M, HosseinKhani S. Mitochondrial distribution and ATP content of vitrified, in vitro matured mouse oocytes. Avicenna J Med Biotechnol. 2014;6(4):210–7.
Eichenlaub-Ritter U, Wieczorek M, Lüke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11(5):783–96.
Gaziev AI, Abdullaev S, Podlutsky A. Mitochondrial function and mitochondrial DNA maintenance with advancing age. Biogerontology. 2014;15(5):417–38.
Nohales-Córcoles M, Sevillano-Almerich G, Di Emidio G, Tatone C, Cobo AC, Dumollard R, et al. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum Reprod. 2016;31(8):1850–8.
Amoushahi M, Salehnia M, Mowla SJ. Vitrification of mouse MII oocyte decreases the mitochondrial DNA copy number, TFAM gene expression and mitochondrial enzyme activity. J Reprod Infertil. 2017;18(4):343–51.
de los Santos MJ, Diez Juan A, Mifsud A, Mercader A, Meseguer M, Rubio C, et al. Variables associated with mitochondrial copy number in human blastocysts: what can we learn from trophectoderm biopsies? Fertil Steril. 2018;109(1):110–7.
La Marca A, Grisendi V, Giulini S, Argento C, Tirelli A, Dondi G, et al. Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovarian Res. 2013;6(1):11.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007 Jan;67(1):73–80.
Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993 Jul;8(7):1061–6.
De Munck N, Santos-Ribeiro S, Mateizel I, Verheyen G. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet. 2015 Sep;32(9):1365–70.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999 Jun;11(3):307–11.
Cuevas Saiz I, Carme Pons Gatell M, Vargas MC, Delgado Mendive A, Rives Enedáguila N, Moragas Solanes M, et al. The embryology interest group: updating ASEBIR’s morphological scoring system for early embryos, morulae and blastocysts. Medicina Reproductiva y Embriología Clínica. 2018;5(1):42–54.
Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, Fragouli E, et al. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51(8):553–62.
Kung A, Munné S, Bankowski B, Coates A, Wells D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod BioMed Online. 2015;31(6):760–9.
Phillips NR, Sprouse ML, Roby RK. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: a multiplex real-time PCR assay. Sci Rep. 2015;4(1):3887.
Gunnala V, Schattman G. Oocyte vitrification for elective fertility preservation: the past, present, and future. Curr Opin Obstet Gynecol. 2017;29(1):59–63.
Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod. 2010;25(5):1199–205.
Almodin CG, Minguetti-Camara VC, Paixao CL, Pereira PC. Embryo development and gestation using fresh and vitrified oocytes. Hum Reprod. 2010;25(5):1192–8.
Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh “versus” vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66–73.
Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21(2):209–27.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–85.
Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertility and Sterility. 2017;107(1):34–42.e3.
May-Panloup P, Boucret L, Chao de la Barca J-M, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–43.
St. John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488–509.
Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, et al. Embryonal mitochondrial DNA: relationship to embryo quality and transfer outcomes. J Assist Reprod Genet. 2018;35(5):871–7.
Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;23:1–9.
Ho JR, Arrach N, Rhodes-Long K, Salem W, McGinnis LK, Chung K, et al. Blastulation timing is associated with differential mitochondrial content in euploid embryos. J Assist Reprod Genet. 2018;35(4):711–20.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertility and Sterility. 2015;104(3):534–541.e1.
Viotti M, Zouves C, Brake A, Tyndall J, Victor A, Griffin D, et al. Blastocysts with disproportionally high mtDNA copy number can result in healthy babies. Reprod BioMed Online. 2019 Apr;38:e25–6.
Lledo B, Ortiz JA, Morales R, García-Hernández E, Ten J, Bernabeu A, et al. Comprehensive mitochondrial DNA analysis and IVF outcome. Human Reproduction Open [Internet]. 2018 Sep 1 [cited 2019 Sep 3];2018(4). Available from: https://academic.oup.com/hropen/article/doi/10.1093/hropen/hoy023/5253765
De Munck N, Vajta G. Safety and efficiency of oocyte vitrification. Cryobiology. 2017;78:119–27.
Acknowledgments
We would like to thank Mr. Victor Lozoya for his help with the statistical interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approval for this study was obtained from the local Ethical Committee of IVIRMA Middle East Fertility Clinic, Abu Dhabi, UAE (Research Ethics Committee REFA029/2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arnanz, A., De Munck, N., Bayram, A. et al. Blastocyst mitochondrial DNA (mtDNA) is not affected by oocyte vitrification: a sibling oocyte study. J Assist Reprod Genet 37, 1387–1397 (2020). https://doi.org/10.1007/s10815-020-01795-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01795-6