Abstract
Purpose
The objective of this study is to analyze the potential of immature, denuded, post-GVBD (germinal vesicle breakdown) oocytes (including prometaphase I, metaphase I, and prometaphase II stages) to result live birth after in vitro maturation. Furthermore, we compared two culture media to identify which of them provides better reproductive outcomes when used for in vitro maturation.
Methods
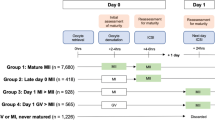
We performed a retrospective cohort study including 4022 IVF-ICSI (in vitro fertilization-intracytoplasmic sperm injection) cycles between 2011 and 2015. A total of 4450 immature post-GVBD oocytes from 1442 cycles were cultured in vitro; of these, 2364 oocytes reached MII (metaphase II) stage (IVMC oocytes, in vitro meiotic completion) and were fertilized. Overall, 3933 embryo transfers were performed: 3579 were embryos derived from MII oocytes (ET-MII); 264 were embryos derived from MII + IVMC oocytes (ET-MIX), and 90 embryos from IVMC oocytes (ET-IVMC). In total, 399 IVMC embryos were transferred.
Results
Maturation rate for immature post-GVBD oocytes was 54.1%. G-2™PLUS (Vitrolife) medium provided significantly higher maturation rate (p < 0.001) than G-IVF™PLUS (Vitrolife) (65.7% vs. 42.5%, p < 0.001). Embryos in ET-IVMC in cleavage stage had an average morphological score of 6.8/10 (7.7 in ET-MII; p < 0.001). Regarding reproductive outcomes, ET-IVMC gave 11.1% biochemical pregnancy rate, 10.0% clinical pregnancy rate, 7.8% ongoing pregnancy rate, and 5.6% live birth rate.
Conclusions
Embryos arising from IVMC oocytes resulted in a live birth rate of 5.6%. We suggest that in vitro maturation of denuded immature post-GVBD oocytes should be performed at the very least when few MII are collected, and likely in all patients, as they provide acceptable maturation and fertilization rates, and a sizeable increase in live birth.


Similar content being viewed by others
References
Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod BioMed Online. 2004;8(2):148–66.
Smith SD, Mikkelsen A, Lindenberg S. Development of human oocytes matured in vitro for 28 or 36 hours. Fertil Steril. 2000;73(3):541–4.
Shin SB, Cho JW, Lee SH, Yang KM, Lim CK, Lee HS. Fertilization and pregnancy potential of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2013;40(1):7–11. https://doi.org/10.5653/cerm.2013.40.1.7.
De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14(7):1859–63.
Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:71. https://doi.org/10.1186/1477-7827-3-71.
Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19(7):1587–90. https://doi.org/10.1093/humrep/deh236.
Balakier H, Sojecki A, Motamedi G, Librach C. Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19(4):982–7. https://doi.org/10.1093/humrep/deh158.
Ko DS, Lee SH, Park DW, Yang KM, Lim CK. Pregnancy and fertilization potential of immature oocytes retrieved in intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2015;42(3):118–25. https://doi.org/10.5653/cerm.2015.42.3.118.
Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Hum Reprod. 2002;17(11):2950–6.
Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod BioMed Online. 2004;8(5):547–52.
Coroleu B, Barri PN, Carreras O, Belil I, Buxaderas R, Veiga A, et al. Effect of using an echogenic catheter for ultrasound-guided embryo transfer in an IVF programme: a prospective, randomized, controlled study. Hum Reprod. 2006;21(7):1809–15. https://doi.org/10.1093/humrep/del045.
Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. https://doi.org/10.1093/aje/kwh090.
Dyrlund TF, Kirkegaard K, Poulsen ET, Sanggaard KW, Hindkjaer JJ, Kjems J, et al. Unconditioned commercial embryo culture media contain a large variety of non-declared proteins: a comprehensive proteomics analysis. Hum Reprod. 2014;29(11):2421–30. https://doi.org/10.1093/humrep/deu220.
Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992;151(2):541–51.
Johnson JE, Higdon HL 3rd, Boone WR. Effect of human granulosa cell co-culture using standard culture media on the maturation and fertilization potential of immature human oocytes. Fertil Steril. 2008;90(5):1674–9. https://doi.org/10.1016/j.fertnstert.2007.06.017.
Jahromi BN, Mosallanezhad Z, Matloob N, Davari M, Ghobadifar MA. The potential role of granulosa cells in the maturation rate of immature human oocytes and embryo development: a co-culture study. Clin Exp Reprod Med. 2015;42(3):111–7. https://doi.org/10.5653/cerm.2015.42.3.111.
Barcena P, Rodriguez M, Obradors A, Vernaeve V, Vassena R. Should we worry about the clock? Relationship between time to ICSI and reproductive outcomes in cycles with fresh and vitrified oocytes. Hum Reprod. 2016;31(6):1182–91. https://doi.org/10.1093/humrep/dew070.
Pujol A, Garcia D, Obradors A, Rodriguez A, Vassena R. Is there a relation between the time to ICSI and the reproductive outcomes? Hum Reprod. 2018;33(5):797–806. https://doi.org/10.1093/humrep/dey067.
Emery BR, Wilcox AL, Aoki VW, Peterson CM, Carrell DT. In vitro oocyte maturation and subsequent delayed fertilization is associated with increased embryo aneuploidy. Fertil Steril. 2005;84(4):1027–9. https://doi.org/10.1016/j.fertnstert.2005.04.036.
Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74(2):295–8.
Acknowledgments
The authors wish to thank Sarai Brazal for her support with the database and Anna Ferrer-Vaquer for critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
N.M.: involved in study design, data analysis, and manuscript preparation. D.G.: involved in study design, data analysis, statistical analysis, and manuscript preparation. R.V. and A.R.: involved in study design, implementation, supervision, expert knowledge, and manuscript preparation.
Corresponding author
Ethics declarations
Ethical approval for performing this study was obtained from the institutional Ethics Committee for Clinical Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(XLS 26 kb)
Supplementary Table 2
(DOC 57 kb)
Rights and permissions
About this article
Cite this article
Martin-Palomino Olid, N., García, D., Rodríguez, A. et al. Could fertility clinics offer a sizable improvement of live birth rates by maturing post-GVBD oocytes in vitro?. J Assist Reprod Genet 36, 1927–1934 (2019). https://doi.org/10.1007/s10815-019-01540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01540-8